 Found 12 hits of kd data for polymerid = 50005810,5360
Found 12 hits of kd data for polymerid = 50005810,5360 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to VDR receptor |
J Med Chem 44: 281-97 (2001)
BindingDB Entry DOI: 10.7270/Q2NP2541 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50102248
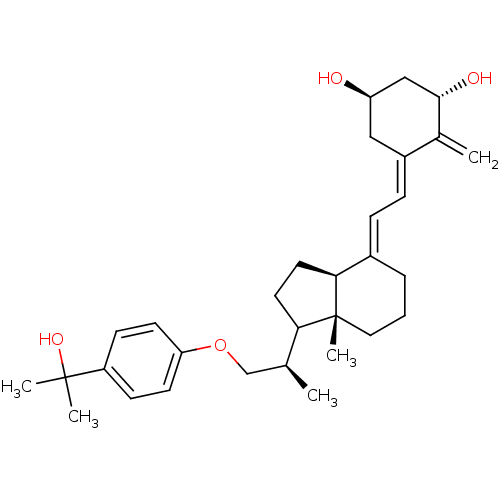
(5-[2-(1-{2-[4-(1-Hydroxy-1-methyl-ethyl)-phenoxy]-...)Show SMILES C[C@@H](COc1ccc(cc1)C(C)(C)O)C1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C31H44O4/c1-20(19-35-26-12-10-24(11-13-26)30(3,4)34)27-14-15-28-22(7-6-16-31(27,28)5)8-9-23-17-25(32)18-29(33)21(23)2/h8-13,20,25,27-29,32-34H,2,6-7,14-19H2,1,3-5H3/b22-8+,23-9-/t20-,25+,27?,28-,29-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | 0.0730 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to VDR receptor |
J Med Chem 44: 281-97 (2001)
BindingDB Entry DOI: 10.7270/Q2NP2541 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50102251

(5-[2-(1-{2-[4-(1-Hydroxy-1-methyl-ethyl)-phenylsul...)Show SMILES C[C@@H](CSc1ccc(cc1)C(C)(C)O)C1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C Show InChI InChI=1S/C31H44O3S/c1-20(19-35-26-12-10-24(11-13-26)30(3,4)34)27-14-15-28-22(7-6-16-31(27,28)5)8-9-23-17-25(32)18-29(33)21(23)2/h8-13,20,25,27-29,32-34H,2,6-7,14-19H2,1,3-5H3/b22-8+,23-9-/t20-,25+,27?,28-,29-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to VDR receptor |
J Med Chem 44: 281-97 (2001)
BindingDB Entry DOI: 10.7270/Q2NP2541 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50369964
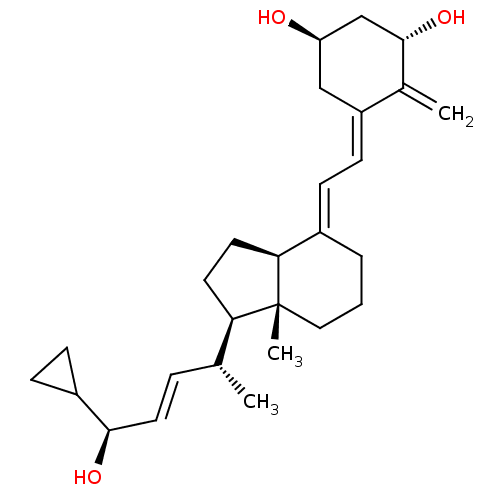
(CALCIPOTRIENE | Calcipotriol)Show SMILES C[C@H](\C=C\[C@@H](O)C1CC1)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H40O3/c1-17(6-13-25(29)20-8-9-20)23-11-12-24-19(5-4-14-27(23,24)3)7-10-21-15-22(28)16-26(30)18(21)2/h6-7,10,13,17,20,22-26,28-30H,2,4-5,8-9,11-12,14-16H2,1,3H3/b13-6+,19-7+,21-10-/t17-,22-,23-,24+,25-,26+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PubMed
| n/a | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to VDR receptor |
J Med Chem 44: 281-97 (2001)
BindingDB Entry DOI: 10.7270/Q2NP2541 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50242220
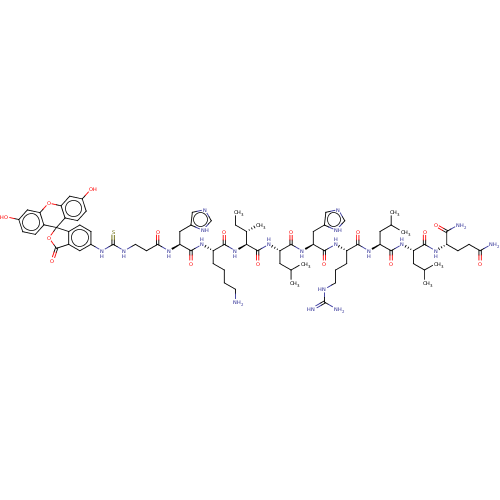
(CHEMBL4066573)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CCNC(=S)Nc1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O |r| Show InChI InChI=1S/C77H109N21O16S/c1-9-42(8)64(73(111)97-57(29-41(6)7)70(108)96-59(32-45-36-84-38-88-45)72(110)93-54(14-12-25-85-75(81)82)66(104)94-56(28-40(4)5)69(107)95-55(27-39(2)3)68(106)91-52(65(80)103)21-22-62(79)101)98-67(105)53(13-10-11-24-78)92-71(109)58(31-44-35-83-37-87-44)90-63(102)23-26-86-76(115)89-43-15-18-49-48(30-43)74(112)114-77(49)50-19-16-46(99)33-60(50)113-61-34-47(100)17-20-51(61)77/h15-20,30,33-42,52-59,64,99-100H,9-14,21-29,31-32,78H2,1-8H3,(H2,79,101)(H2,80,103)(H,83,87)(H,84,88)(H,90,102)(H,91,106)(H,92,109)(H,93,110)(H,94,104)(H,95,107)(H,96,108)(H,97,111)(H,98,105)(H4,81,82,85)(H2,86,89,115)/t42-,52-,53-,54-,55-,56-,57-,58-,59-,64-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a |
Shenzhen Graduate School of Peking University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged VDR LBD (156 to 453 residues) expressed in Echerichia coli BL21 star (DE3) after 40 mins in presence... |
J Med Chem 60: 8731-8740 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00732
BindingDB Entry DOI: 10.7270/Q2BP056C |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50242218

(CHEMBL4086339)Show SMILES CC[C@H](C)[C@@H]1NC(=O)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NCCC(=O)NC(=S)Nc2ccc3c(c2)C(=O)OC32c3ccc(O)cc3Oc3cc(O)ccc23)C(=O)NCC(NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O |r| Show InChI InChI=1S/C72H104N20O17S/c1-9-37(8)58-67(106)89-50(28-36(6)7)65(104)90-52(66(105)85-47(13-11-24-81-70(77)78)62(101)86-49(27-35(4)5)64(103)87-48(26-34(2)3)63(102)84-45(59(74)98)20-21-55(73)95)33-82-60(99)51(32-57(97)91-58)88-61(100)46(12-10-23-80-69(75)76)79-25-22-56(96)92-71(110)83-38-14-17-42-41(29-38)68(107)109-72(42)43-18-15-39(93)30-53(43)108-54-31-40(94)16-19-44(54)72/h14-19,29-31,34-37,45-52,58,79,93-94H,9-13,20-28,32-33H2,1-8H3,(H2,73,95)(H2,74,98)(H,82,99)(H,84,102)(H,85,105)(H,86,101)(H,87,103)(H,88,100)(H,89,106)(H,90,104)(H,91,97)(H4,75,76,80)(H4,77,78,81)(H2,83,92,96,110)/t37-,45-,46-,47-,48-,49-,50-,51-,52?,58-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a |
Shenzhen Graduate School of Peking University
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged VDR LBD (156 to 453 residues) expressed in Echerichia coli BL21 star (DE3) after 40 mins in presence... |
J Med Chem 60: 8731-8740 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00732
BindingDB Entry DOI: 10.7270/Q2BP056C |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50236238
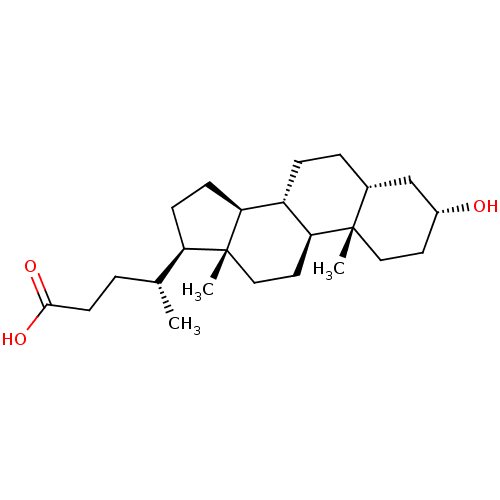
((3alpha,5beta)-3-hydroxycholan-24-oic acid | 3alph...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C24H40O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h15-21,25H,4-14H2,1-3H3,(H,26,27)/t15-,16-,17-,18+,19-,20+,21+,23+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal His-tagged human VDR LBD canonical site (118 to 427) by direct isothermal titration calorimetric analysis |
J Med Chem 57: 4710-9 (2014)
Article DOI: 10.1021/jm5002524
BindingDB Entry DOI: 10.7270/Q2PV6MX8 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50242219
(CHEMBL4067602)Show SMILES CC[C@H](C)[C@@H]1NC(=O)C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CCNC(=S)Nc2ccc3c(c2)C(=O)OC32c3ccc(O)cc3Oc3cc(O)ccc23)C(=O)NCC(NC(=O)C(CC(C)(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(CC(C)(C)C)C(=O)NC(CC(C)(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O |r| Show InChI InChI=1S/C75H110N20O17S/c1-12-37(2)58-67(109)93-51(35-74(9,10)11)65(107)94-52(66(108)89-47(16-14-27-83-70(80)81)62(104)91-50(34-73(6,7)8)64(106)92-49(33-72(3,4)5)63(105)88-45(59(77)101)23-24-55(76)98)36-85-60(102)48(32-57(100)95-58)90-61(103)46(15-13-26-82-69(78)79)87-56(99)25-28-84-71(113)86-38-17-20-42-41(29-38)68(110)112-75(42)43-21-18-39(96)30-53(43)111-54-31-40(97)19-22-44(54)75/h17-22,29-31,37,45-52,58,96-97H,12-16,23-28,32-36H2,1-11H3,(H2,76,98)(H2,77,101)(H,85,102)(H,87,99)(H,88,105)(H,89,108)(H,90,103)(H,91,104)(H,92,106)(H,93,109)(H,94,107)(H,95,100)(H4,78,79,82)(H4,80,81,83)(H2,84,86,113)/t37-,45-,46-,47-,48-,49?,50?,51?,52?,58-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Shenzhen Graduate School of Peking University
Curated by ChEMBL
| Assay Description
Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation |
J Med Chem 60: 8731-8740 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00732
BindingDB Entry DOI: 10.7270/Q2BP056C |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50236238

((3alpha,5beta)-3-hydroxycholan-24-oic acid | 3alph...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C24H40O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h15-21,25H,4-14H2,1-3H3,(H,26,27)/t15-,16-,17-,18+,19-,20+,21+,23+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.52E+3 | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal His-tagged human VDR LBD low-affinity site (118 to 427) by direct isothermal titration calorimetric analysis |
J Med Chem 57: 4710-9 (2014)
Article DOI: 10.1021/jm5002524
BindingDB Entry DOI: 10.7270/Q2PV6MX8 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50461063
(CHEMBL4226803 | US10457637, Compound 8)Show SMILES CCc1ccc(cc1)N(CC(C)C)S(=O)(=O)c1ccc(OCC2CCOCC2)c(c1)[S@@](C)(=N)=O |r| Show InChI InChI=1S/C25H36N2O5S2/c1-5-20-6-8-22(9-7-20)27(17-19(2)3)34(29,30)23-10-11-24(25(16-23)33(4,26)28)32-18-21-12-14-31-15-13-21/h6-11,16,19,21,26H,5,12-15,17-18H2,1-4H3/t33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Activity at VDR expressed in human HeLa-derived HDLN6 cells assessed as inhibition of transcriptional activity after 18 hrs by luminescence-based luc... |
Bioorg Med Chem Lett 28: 1269-1273 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.041
BindingDB Entry DOI: 10.7270/Q2WD437Q |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50203462

(CHEMBL3931161)Show SMILES CN(C)C(=O)c1ccc2n(nc(Oc3c(Cl)cccc3C(F)(F)F)c2c1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H17ClF3N3O4/c1-30(2)22(32)14-8-11-19-16(12-14)21(29-31(19)15-9-6-13(7-10-15)23(33)34)35-20-17(24(26,27)28)4-3-5-18(20)25/h3-12H,1-2H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Galderma R&D
Curated by ChEMBL
| Assay Description
Activity at 24 hydroxylase-fused VDR in human HG5LN cells after 18 hrs by luciferase reporter gene assay |
Bioorg Med Chem Lett 26: 5802-5808 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.023
BindingDB Entry DOI: 10.7270/Q2D79DDH |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50236238

((3alpha,5beta)-3-hydroxycholan-24-oic acid | 3alph...)Show SMILES C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C24H40O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h15-21,25H,4-14H2,1-3H3,(H,26,27)/t15-,16-,17-,18+,19-,20+,21+,23+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal His-tagged human VDR LBD (118 to 427) by reverse isothermal titration calorimetric analysis |
J Med Chem 57: 4710-9 (2014)
Article DOI: 10.1021/jm5002524
BindingDB Entry DOI: 10.7270/Q2PV6MX8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data