

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-O (Homo sapiens) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gachon University Curated by ChEMBL | Assay Description Inhibition of human CDK2/cyclin-O using histone H1 as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 163: 453-480 (2019) Article DOI: 10.1016/j.ejmech.2018.11.037 BindingDB Entry DOI: 10.7270/Q2TQ650N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-O (Homo sapiens) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gachon University Curated by ChEMBL | Assay Description Inhibition of human CDK2/cyclin-O using histone H1 as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 163: 453-480 (2019) Article DOI: 10.1016/j.ejmech.2018.11.037 BindingDB Entry DOI: 10.7270/Q2TQ650N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-O (Homo sapiens) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CDK2/cyclin-O using histone H1 as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-O (Homo sapiens) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin-O (unknown origin) using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113232 BindingDB Entry DOI: 10.7270/Q22J6GMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-O (Homo sapiens) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human CDK2/cyclin-O using histone H1 as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-O (Homo sapiens) | BDBM50563174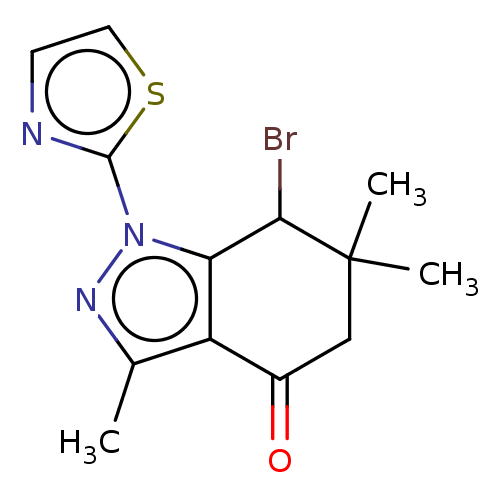 (CHEMBL1708376) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin-O (unknown origin) using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113232 BindingDB Entry DOI: 10.7270/Q22J6GMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-O (Homo sapiens) | BDBM50563177 (CHEMBL4763182) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin-O (unknown origin) using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113232 BindingDB Entry DOI: 10.7270/Q22J6GMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-O (Homo sapiens) | BDBM50563140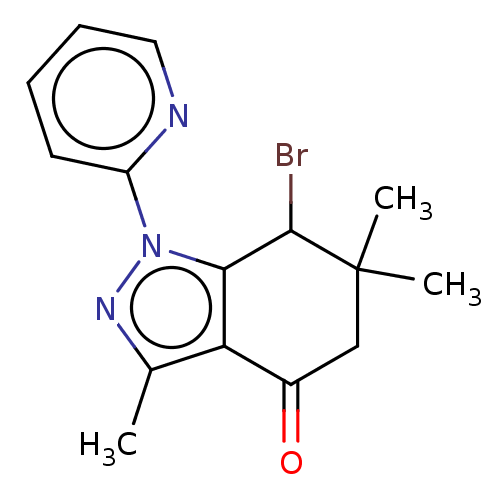 (CHEMBL1906448) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin-O (unknown origin) using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113232 BindingDB Entry DOI: 10.7270/Q22J6GMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-O/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50263013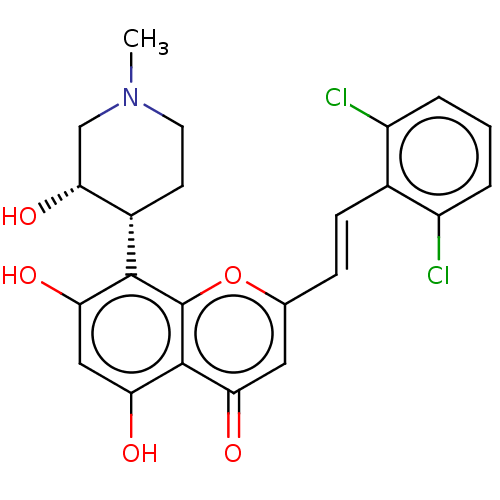 (CHEMBL4079206) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry Division, CSIR-Indian Institute of Integrative Medicine , Canal Road, Jammu-180001, India. Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin O (unknown origin) after 30 mins in presence of [33P]-gamma-ATP by filter binding assay | J Med Chem 61: 1664-1687 (2018) Article DOI: 10.1021/acs.jmedchem.7b01765 BindingDB Entry DOI: 10.7270/Q2DV1NC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||