

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingomyelin phosphodiesterase (Homo sapiens) | BDBM50407246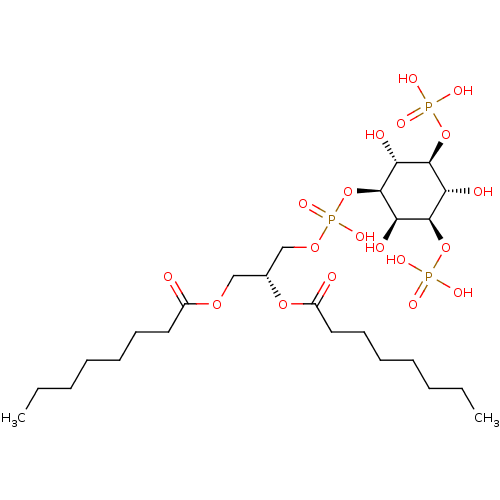 (CHEMBL5279916) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Negative log concentration of antagonistic compound was determined on 5-hydroxytryptamine 2B receptor of Rat stomach fundus | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase (Homo sapiens) | BDBM50520425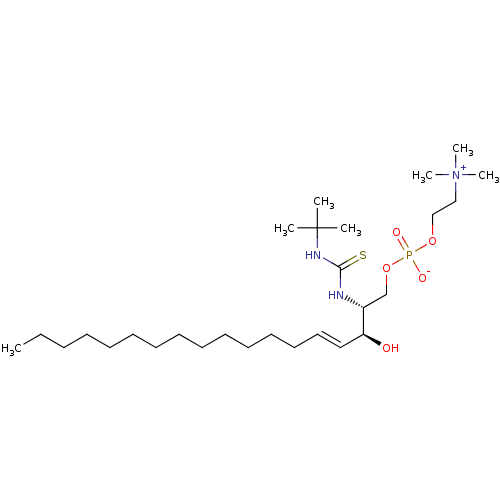 (CHEMBL4556453) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of acid sphingomyelinase (unknown origin) | J Med Chem 63: 961-974 (2020) Article DOI: 10.1021/acs.jmedchem.9b00739 BindingDB Entry DOI: 10.7270/Q2FJ2M5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||