

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Human rhinovirus 14) | BDBM50455106 (CHEMBL4216095) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455115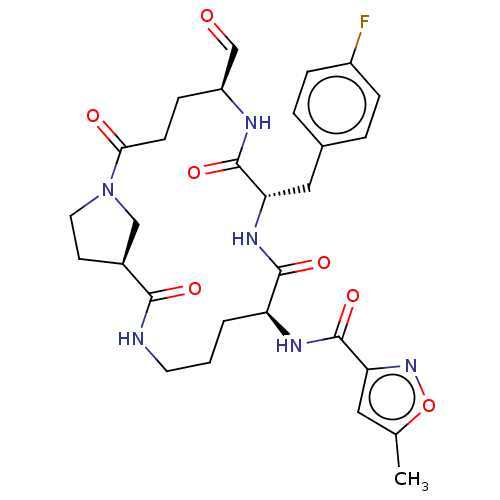 (CHEMBL4202932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455107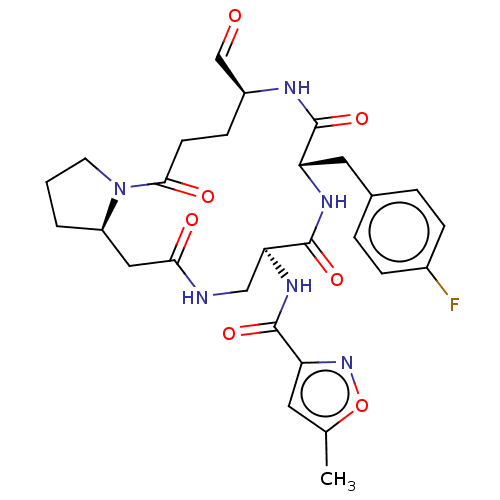 (CHEMBL4212167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455105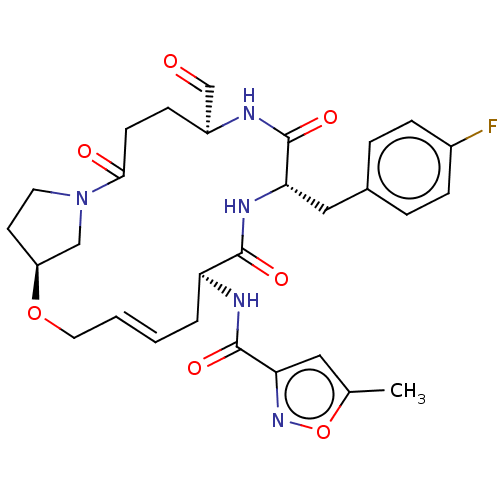 (CHEMBL4218384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455114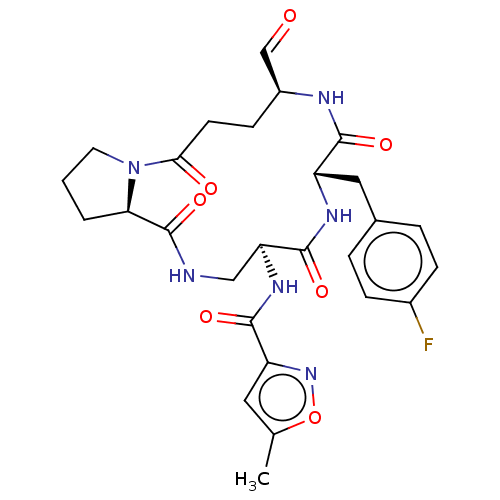 (CHEMBL4207862) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455112 (CHEMBL4215964) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455113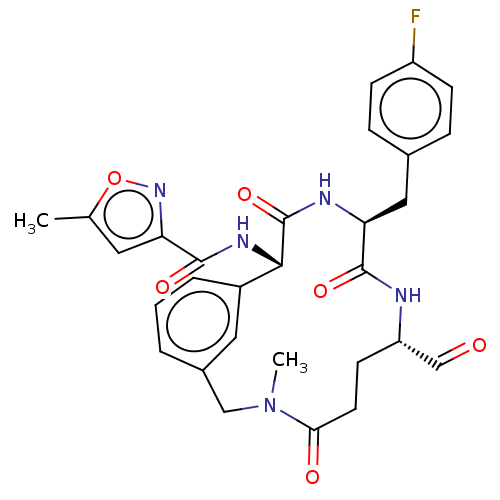 (CHEMBL4212963) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455111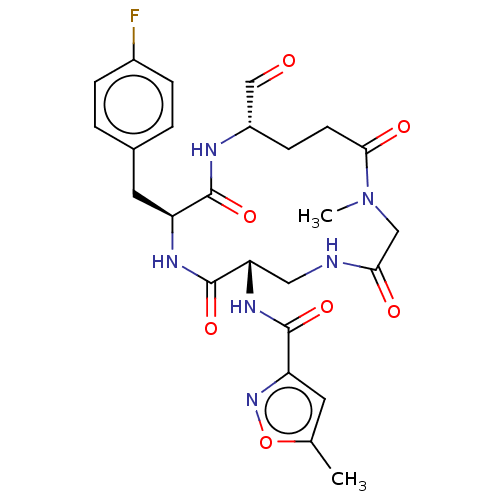 (CHEMBL4217566) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455110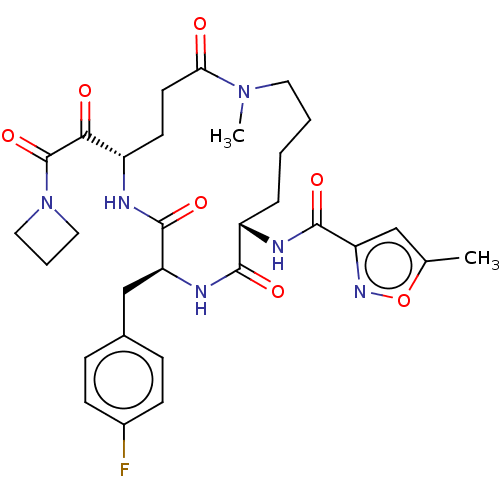 (CHEMBL4203502) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455104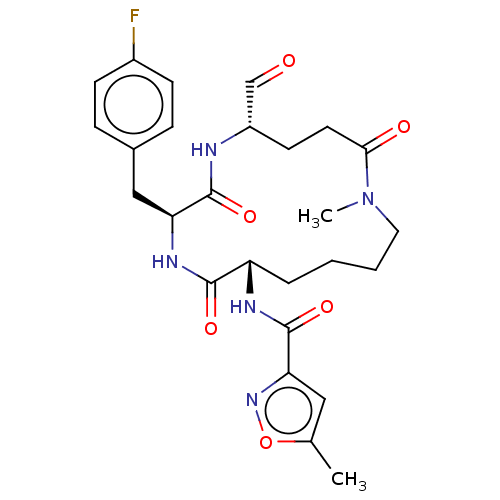 (CHEMBL4211003) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455108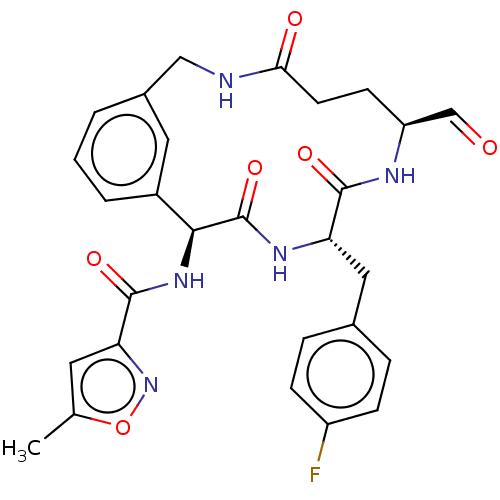 (CHEMBL4207281) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 753 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50462014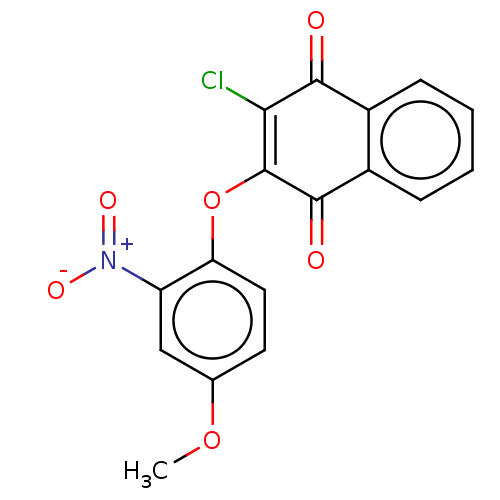 (CHEMBL4224853) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human rhinovirus protease 3C using fluorogenic-Dabcyl-KTSAVLQSGFRKME-Edan as substrate after 5 mins by FRET assay | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455102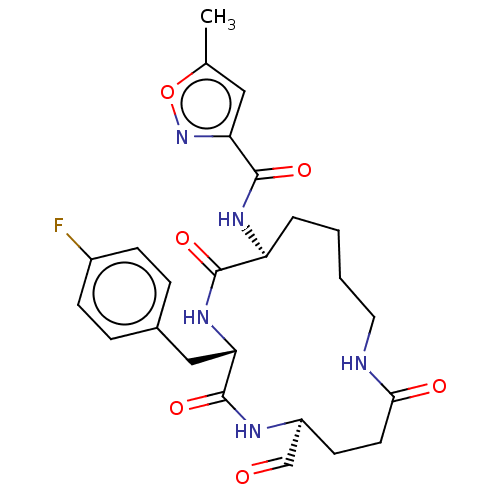 (CHEMBL4206628) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455109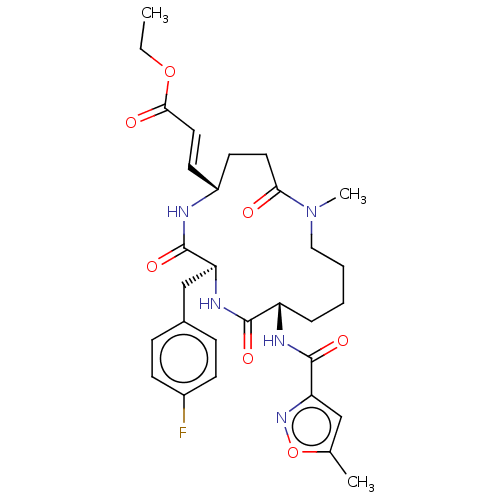 (CHEMBL4207200) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50455103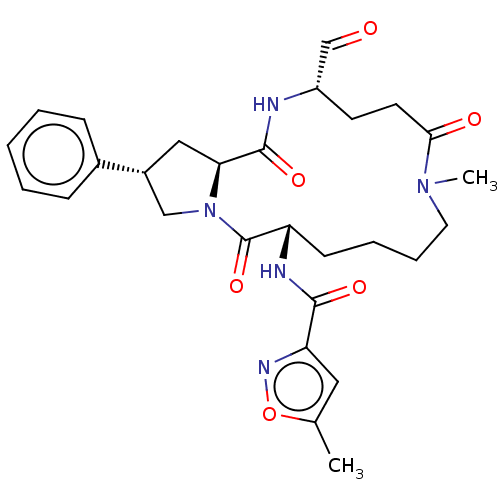 (CHEMBL4202850) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra... | Bioorg Med Chem Lett 28: 906-909 (2018) Article DOI: 10.1016/j.bmcl.2018.01.064 BindingDB Entry DOI: 10.7270/Q2TF00Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus 14) | BDBM50462026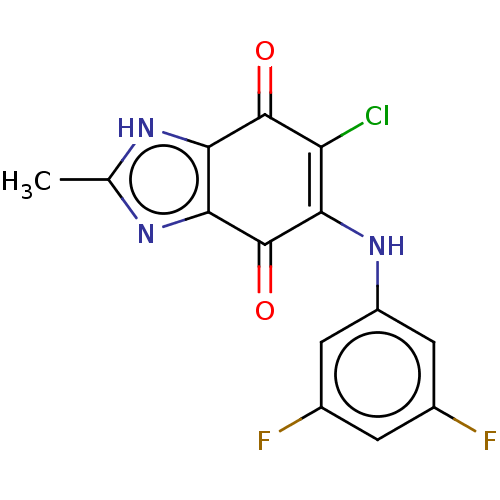 (CHEMBL4229177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human rhinovirus protease 3C using fluorogenic-Dabcyl-KTSAVLQSGFRKME-Edan as substrate after 5 mins by FRET assay | Bioorg Med Chem Lett 28: 2533-2538 (2018) Article DOI: 10.1016/j.bmcl.2018.05.046 BindingDB Entry DOI: 10.7270/Q2HT2S0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||