 Found 249 hits of ic50 for UniProtKB: P36544
Found 249 hits of ic50 for UniProtKB: P36544 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50170589
((3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2...)Show SMILES C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CNC(=O)CN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H97N23O24S4/c1-27(75-61(108)42(24-115)86-54(101)29(3)76-64(111)44-5-4-12-88(44)65(112)38(14-31-19-71-26-73-31)83-60(107)39(21-89)84-63(110)43(25-116)87-62(109)41(23-114)77-49(95)20-72-48(94)18-66)52(99)74-28(2)53(100)79-35(15-46(68)92)58(105)81-36(16-47(69)93)57(104)78-33(10-11-45(67)91)55(102)82-37(17-50(96)97)59(106)80-34(13-30-6-8-32(90)9-7-30)56(103)85-40(22-113)51(70)98/h6-9,19,26-29,33-44,89-90,113-116H,4-5,10-18,20-25,66H2,1-3H3,(H2,67,91)(H2,68,92)(H2,69,93)(H2,70,98)(H,71,73)(H,72,94)(H,74,99)(H,75,108)(H,76,111)(H,77,95)(H,78,104)(H,79,100)(H,80,106)(H,81,105)(H,82,102)(H,83,107)(H,84,110)(H,85,103)(H,86,101)(H,87,109)(H,96,97)/t27-,28-,29-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha 7 |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50170586
((3S)-3-{[(2S)-1-[(2S)-2-[(2S,3R)-2-[(2S)-2-[(2S)-2...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CS)C=O Show InChI InChI=1S/C65H92N16O21S4/c1-32(53(90)78-52(33(2)84)62(99)74-41(23-49(67)86)64(101)79-18-6-11-46(79)60(97)72-39(24-51(88)89)55(92)69-36(26-82)28-103)68-54(91)38(21-34-9-4-3-5-10-34)71-58(95)45(31-106)77-61(98)47-12-7-19-80(47)65(102)48-13-8-20-81(48)63(100)40(22-35-14-16-37(85)17-15-35)73-56(93)42(27-83)75-59(96)44(30-105)76-57(94)43(29-104)70-50(87)25-66/h3-5,9-10,14-17,26,32-33,36,38-48,52,83-85,103-106H,6-8,11-13,18-25,27-31,66H2,1-2H3,(H2,67,86)(H,68,91)(H,69,92)(H,70,87)(H,71,95)(H,72,97)(H,73,93)(H,74,99)(H,75,96)(H,76,94)(H,77,98)(H,78,90)(H,88,89)/t32-,33+,36+,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,52-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha7; Range is 0.3-1.5 nM |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50393253
(CHEMBL2151573)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]2CSSC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)[C@@H]3CCCN3C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N2 |r| Show InChI InChI=1S/C89H143N37O31S4/c1-38(2)24-47-72(143)115-49(27-60(91)128)74(145)118-51(28-61(92)129)83(154)126-23-9-15-59(126)82(153)117-48(25-40-31-102-37-107-40)73(144)116-50(30-65(135)136)75(146)123-55(78(149)111-42(10-4-18-103-86(94)95)68(139)110-43(11-5-19-104-87(96)97)69(140)113-46(85(156)157)13-7-21-106-89(100)101)34-159-160-35-56-79(150)120-53(32-127)76(147)119-52(29-62(93)130)84(155)125-22-8-14-58(125)81(152)108-39(3)66(137)121-54(77(148)112-44(70(141)114-47)12-6-20-105-88(98)99)33-158-161-36-57(80(151)124-56)122-71(142)45(16-17-63(131)132)109-67(138)41(90)26-64(133)134/h31,37-39,41-59,127H,4-30,32-36,90H2,1-3H3,(H2,91,128)(H2,92,129)(H2,93,130)(H,102,107)(H,108,152)(H,109,138)(H,110,139)(H,111,149)(H,112,148)(H,113,140)(H,114,141)(H,115,143)(H,116,144)(H,117,153)(H,118,145)(H,119,147)(H,120,150)(H,121,137)(H,122,142)(H,123,146)(H,124,151)(H,131,132)(H,133,134)(H,135,136)(H,156,157)(H4,94,95,103)(H4,96,97,104)(H4,98,99,105)(H4,100,101,106)/t39-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09 | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha7 nAChR expressed in Xenopus oocyte |
J Med Chem 54: 7943-61 (2011)
Article DOI: 10.1021/jm2007672
BindingDB Entry DOI: 10.7270/Q29P32QP |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50140089
(CHEMBL437423 | GCCSHPACAGNNQHIC*)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C61H96N24O20S4/c1-5-26(2)47(60(104)81-37(20-106)48(66)92)84-54(98)32(11-29-16-67-24-70-29)77-51(95)31(8-9-42(63)87)76-53(97)34(14-44(65)89)78-52(96)33(13-43(64)88)74-46(91)18-69-49(93)27(3)72-56(100)39(22-108)82-50(94)28(4)73-59(103)41-7-6-10-85(41)61(105)35(12-30-17-68-25-71-30)79-55(99)36(19-86)80-58(102)40(23-109)83-57(101)38(21-107)75-45(90)15-62/h16-17,24-28,31-41,47,86,106-109H,5-15,18-23,62H2,1-4H3,(H2,63,87)(H2,64,88)(H2,65,89)(H2,66,92)(H,67,70)(H,68,71)(H,69,93)(H,72,100)(H,73,103)(H,74,91)(H,75,90)(H,76,97)(H,77,95)(H,78,96)(H,79,99)(H,80,102)(H,81,104)(H,82,94)(H,83,101)(H,84,98)/t26-,27-,28-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,47-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha 7 |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50597512

(CHEMBL5201239)Show SMILES [I-].C[N+]1(C)CC[C@@H](C1)Oc1ccc(\C=C\c2ccccc2)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00746
BindingDB Entry DOI: 10.7270/Q2J96BDG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50597513

(CHEMBL5178473)Show SMILES [I-].C[N+]1(C)CC[C@H](C1)Oc1ccc(\C=C\c2ccccc2)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00746
BindingDB Entry DOI: 10.7270/Q2J96BDG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50170592
(CHEMBL411146)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C=O Show InChI InChI=1S/C74H124N26O20S4/c1-36(2)26-45(62(111)91-46(27-40-17-19-42(104)20-18-40)63(112)94-48(32-121)59(108)85-29-55(106)88-43(13-8-22-83-73(78)79)61(110)90-44(14-9-23-84-74(80)81)60(109)87-41(30-101)12-7-21-82-72(76)77)92-69(118)56(37(3)4)97-58(107)38(5)86-65(114)50(34-123)96-68(117)52-15-10-24-99(52)70(119)53-16-11-25-100(53)71(120)57(39(6)103)98-64(113)47(31-102)93-67(116)51(35-124)95-66(115)49(33-122)89-54(105)28-75/h17-20,30,36-39,41,43-53,56-57,102-104,121-124H,7-16,21-29,31-35,75H2,1-6H3,(H,85,108)(H,86,114)(H,87,109)(H,88,106)(H,89,105)(H,90,110)(H,91,111)(H,92,118)(H,93,116)(H,94,112)(H,95,115)(H,96,117)(H,97,107)(H,98,113)(H4,76,77,82)(H4,78,79,83)(H4,80,81,84)/t38-,39+,41-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,56-,57-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha7; Range is 0.3-1.5 nM |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50597506

(CHEMBL5190813)Show SMILES [I-].C[N+]1(CCOc2ccc(\C=C\c3ccccc3)cc2)CCCC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00746
BindingDB Entry DOI: 10.7270/Q2J96BDG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM80642
(CHEMBL191491 | MG 624 | MLS002172460 | SMR00125409...)Show InChI InChI=1S/C22H30NO/c1-4-23(5-2,6-3)18-19-24-22-16-14-21(15-17-22)13-12-20-10-8-7-9-11-20/h7-17H,4-6,18-19H2,1-3H3/q+1/b13-12+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00746
BindingDB Entry DOI: 10.7270/Q2J96BDG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50597507

(CHEMBL5176101)Show SMILES [I-].C[N+]1(CCOc2ccc(\C=C\c3ccccc3)cc2)CCCCC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00746
BindingDB Entry DOI: 10.7270/Q2J96BDG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50597505
(CHEMBL5174508)Show SMILES [I-].C[N+]1(CCOc2ccc(\C=C\c3ccccc3)cc2)CCC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00746
BindingDB Entry DOI: 10.7270/Q2J96BDG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50597504

(CHEMBL5177606)Show SMILES [I-].C(C[N+]12CCC(CC1)CC2)Oc1ccc(\C=C\c2ccccc2)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00746
BindingDB Entry DOI: 10.7270/Q2J96BDG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50170584
(CHEMBL411145)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)NC(CCC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CC(O)CC1C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C83H137N31O28S4/c1-8-36(6)60(87)77(138)100-40(12-9-17-94-82(89)90)65(126)101-45(25-59(122)123)69(130)99-42(15-16-58(120)121)66(127)109-52(32-146)74(135)110-51(31-145)73(134)106-48(28-115)71(132)105-46(23-56(85)118)80(141)113-19-11-14-53(113)75(136)97-37(7)64(125)108-50(30-144)72(133)98-41(13-10-18-95-83(91)92)67(128)111-61(34(2)3)78(139)103-44(22-55(84)117)68(129)104-47(24-57(86)119)81(142)114-27-39(116)21-54(114)76(137)102-43(20-38-26-93-33-96-38)70(131)112-62(35(4)5)79(140)107-49(29-143)63(88)124/h26,33-37,39-54,60-62,115-116,143-146H,8-25,27-32,87H2,1-7H3,(H2,84,117)(H2,85,118)(H2,86,119)(H2,88,124)(H,93,96)(H,97,136)(H,98,133)(H,99,130)(H,100,138)(H,101,126)(H,102,137)(H,103,139)(H,104,129)(H,105,132)(H,106,134)(H,107,140)(H,108,125)(H,109,127)(H,110,135)(H,111,128)(H,112,131)(H,120,121)(H,122,123)(H4,89,90,94)(H4,91,92,95)/t36-,37-,39?,40-,41-,42?,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54?,60-,61-,62-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha-7 Range is 3-5 nM |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50175606

(CHEMBL3302240)Show InChI InChI=1S/C27H50N4O8SSi2/c1-18-15-31(24(33)30(22(18)32)14-12-13-28)23-21(38-42(10,11)26(5,6)7)27(19(29)17-40(34,35)39-27)20(37-23)16-36-41(8,9)25(2,3)4/h15,17,20-21,23H,12-14,16,28-29H2,1-11H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Retrophin, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at alpha7 nAChR (unknown origin) |
Bioorg Med Chem Lett 26: 3010-3 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.022
BindingDB Entry DOI: 10.7270/Q2R49SPQ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50466614

(CHEMBL4291079)Show SMILES [I-].CC[N+](CC)(CC)CCCCOc1ccc(\C=C\c2ccccc2)cc1 Show InChI InChI=1S/C24H34NO/c1-4-25(5-2,6-3)20-10-11-21-26-24-18-16-23(17-19-24)15-14-22-12-8-7-9-13-22/h7-9,12-19H,4-6,10-11,20-21H2,1-3H3/q+1/b15-14+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha7 nACHR expressed in xenopous laevis oocyte assessed as inhibition of ACh-induced channel current after 5 mins at -... |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50140087
(CHEMBL265198 | GCCSNPVCHLEHSNLC*)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(C)C)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C67H107N23O22S4/c1-29(2)12-35(55(100)77-34(9-10-51(96)97)54(99)80-38(15-33-20-73-28-75-33)58(103)84-41(21-91)60(105)82-39(16-48(69)93)59(104)79-36(13-30(3)4)56(101)86-43(23-113)53(71)98)78-57(102)37(14-32-19-72-27-74-32)81-63(108)46(26-116)88-66(111)52(31(5)6)89-65(110)47-8-7-11-90(47)67(112)40(17-49(70)94)83-61(106)42(22-92)85-64(109)45(25-115)87-62(107)44(24-114)76-50(95)18-68/h19-20,27-31,34-47,52,91-92,113-116H,7-18,21-26,68H2,1-6H3,(H2,69,93)(H2,70,94)(H2,71,98)(H,72,74)(H,73,75)(H,76,95)(H,77,100)(H,78,102)(H,79,104)(H,80,99)(H,81,108)(H,82,105)(H,83,106)(H,84,103)(H,85,109)(H,86,101)(H,87,107)(H,88,111)(H,89,110)(H,96,97)/t34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,52-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha7; Range is 0.5-8 nM |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50366779
(METHYLLYCACONITINE)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)[C@@]34[C@@H]5C[C@H]6[C@H](OC)[C@@H]5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)[C@H]23)[C@H]14 |r,wU:4.4,48.54,44.48,42.46,36.39,39.43,wD:28.52,35.36,31.32,29.31,47.50,25.27,32.34,TLB:23:4:28:44.42,4:47:36.29.35:48,1:2:28:44.42,45:44:36.29.35:48,44:47:24.23.25:3.48.2,42:36:32:29.30,THB:37:36:32:29.30,40:39:32:29.30,5:4:28:44.42,(5.29,-1.99,;6.48,-1.02,;7.91,-1.57,;7.91,-4.71,;8.6,-3.19,;9.67,-4.27,;9.27,-5.75,;10.35,-6.84,;11.83,-6.45,;9.94,-8.32,;8.46,-8.7,;8.06,-10.18,;9.14,-11.27,;10.63,-10.87,;11.02,-9.39,;12.51,-8.99,;13.7,-9.95,;13.62,-11.48,;14.98,-9.12,;14.58,-7.64,;15.55,-6.45,;13.05,-7.56,;11.52,-7.55,;7.27,-2.43,;7.27,-.88,;8.6,-.11,;8.59,1.42,;7.27,2.19,;9.93,-.88,;11.13,.09,;10.97,1.62,;12.38,2.24,;13.41,1.1,;17.14,1.1,;17.91,-.25,;12.64,-.24,;13.31,-1.62,;14.39,-2.7,;15.18,-1.64,;15.11,2.18,;16.19,3.26,;15.8,4.74,;12.66,-3.02,;13.74,-4.1,;11.15,-3.38,;10.82,-4.87,;12.29,-5.26,;9.94,-2.42,;9.88,1.15,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22-,24+,25+,27-,28+,29-,30+,33-,34+,35-,36+,37+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha-7 nAChR in tsA201 cells coexpressed with Ric3 by FMP assay |
J Med Chem 50: 4616-29 (2007)
Article DOI: 10.1021/jm070574f
BindingDB Entry DOI: 10.7270/Q2J67HRC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50366779

(METHYLLYCACONITINE)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)[C@@]34[C@@H]5C[C@H]6[C@H](OC)[C@@H]5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)[C@H]23)[C@H]14 |r,wU:4.4,48.54,44.48,42.46,36.39,39.43,wD:28.52,35.36,31.32,29.31,47.50,25.27,32.34,TLB:23:4:28:44.42,4:47:36.29.35:48,1:2:28:44.42,45:44:36.29.35:48,44:47:24.23.25:3.48.2,42:36:32:29.30,THB:37:36:32:29.30,40:39:32:29.30,5:4:28:44.42,(5.29,-1.99,;6.48,-1.02,;7.91,-1.57,;7.91,-4.71,;8.6,-3.19,;9.67,-4.27,;9.27,-5.75,;10.35,-6.84,;11.83,-6.45,;9.94,-8.32,;8.46,-8.7,;8.06,-10.18,;9.14,-11.27,;10.63,-10.87,;11.02,-9.39,;12.51,-8.99,;13.7,-9.95,;13.62,-11.48,;14.98,-9.12,;14.58,-7.64,;15.55,-6.45,;13.05,-7.56,;11.52,-7.55,;7.27,-2.43,;7.27,-.88,;8.6,-.11,;8.59,1.42,;7.27,2.19,;9.93,-.88,;11.13,.09,;10.97,1.62,;12.38,2.24,;13.41,1.1,;17.14,1.1,;17.91,-.25,;12.64,-.24,;13.31,-1.62,;14.39,-2.7,;15.18,-1.64,;15.11,2.18,;16.19,3.26,;15.8,4.74,;12.66,-3.02,;13.74,-4.1,;11.15,-3.38,;10.82,-4.87,;12.29,-5.26,;9.94,-2.42,;9.88,1.15,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22-,24+,25+,27-,28+,29-,30+,33-,34+,35-,36+,37+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha7nAChR co-expressed with human Ric3 in tsA201 cells by FMP assay |
J Med Chem 50: 4616-29 (2007)
Article DOI: 10.1021/jm070574f
BindingDB Entry DOI: 10.7270/Q2J67HRC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50339937

(1,1',1''-(5,5',5''-(benzene-1,3,5-triyl)tris(penta...)Show SMILES C(CCc1cc(CCCCC[n+]2cccc(c2)-c2ccccc2)cc(CCCCC[n+]2cccc(c2)-c2ccccc2)c1)CC[n+]1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C54H60N3/c1-10-25-49(26-11-1)52-31-19-37-55(43-52)34-16-4-7-22-46-40-47(23-8-5-17-35-56-38-20-32-53(44-56)50-27-12-2-13-28-50)42-48(41-46)24-9-6-18-36-57-39-21-33-54(45-57)51-29-14-3-15-30-51/h1-3,10-15,19-21,25-33,37-45H,4-9,16-18,22-24,34-36H2/q+3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha7 nAChR expressed in Xenopus oocyte assessed as inhibition of ACh-gated current by voltage clamp electrophysiology assay |
Bioorg Med Chem Lett 21: 2476-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.043
BindingDB Entry DOI: 10.7270/Q2TD9XN9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50170600
((3S)-3-{[(2S)-1-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H99N19O22S4/c1-29(2)18-37(76-57(98)39(24-85)78-60(101)43(28-110)80-59(100)41(26-108)72-49(89)23-66)63(104)84-17-7-10-46(84)65(106)83-16-6-9-45(83)62(103)81-42(27-109)58(99)71-30(3)52(93)70-31(4)53(94)73-35(20-47(67)87)55(96)77-38(21-48(68)88)64(105)82-15-5-8-44(82)61(102)75-36(22-50(90)91)56(97)74-34(19-32-11-13-33(86)14-12-32)54(95)79-40(25-107)51(69)92/h11-14,29-31,34-46,85-86,107-110H,5-10,15-28,66H2,1-4H3,(H2,67,87)(H2,68,88)(H2,69,92)(H,70,93)(H,71,99)(H,72,89)(H,73,94)(H,74,97)(H,75,102)(H,76,98)(H,77,96)(H,78,101)(H,79,95)(H,80,100)(H,81,103)(H,90,91)/t30-,31-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha 7 |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50175589
(CHEMBL3302229)Show InChI InChI=1S/C18H22N4O3/c1-24-16-6-4-14(5-7-16)21-18(23)22-10-9-20-11-15(22)13-25-17-3-2-8-19-12-17/h2-8,12,15,20H,9-11,13H2,1H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Retrophin, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at alpha7 nAChR (unknown origin) |
Bioorg Med Chem Lett 26: 3010-3 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.022
BindingDB Entry DOI: 10.7270/Q2R49SPQ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50466607
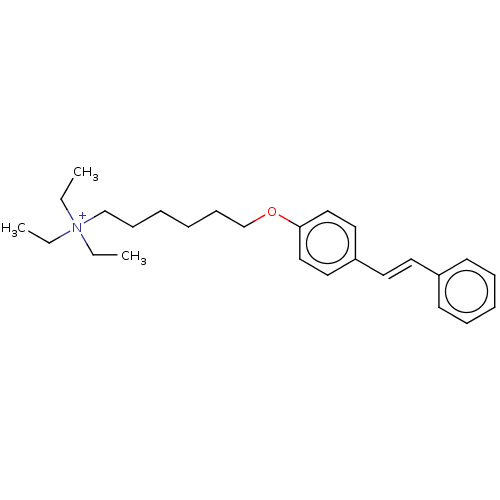
(CHEMBL4293646)Show SMILES [I-].CC[N+](CC)(CC)CCCCCCOc1ccc(\C=C\c2ccccc2)cc1 Show InChI InChI=1S/C26H38NO/c1-4-27(5-2,6-3)22-12-7-8-13-23-28-26-20-18-25(19-21-26)17-16-24-14-10-9-11-15-24/h9-11,14-21H,4-8,12-13,22-23H2,1-3H3/q+1/b17-16+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha7 nACHR expressed in xenopous laevis oocyte assessed as inhibition of ACh-induced channel current after 5 mins at -... |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50175603

(CHEMBL3303977)Show SMILES COc1ccc(NC(=O)N2CCNC[C@@H]2COc2cccnc2)cc1 |r| Show InChI InChI=1S/C28H51N3O9SSi2/c1-19-16-31(25(34)30(23(19)33)14-12-13-15-32)24-22(39-43(10,11)27(5,6)7)28(20(29)18-41(35,36)40-28)21(38-24)17-37-42(8,9)26(2,3)4/h16,18,21-22,24,32H,12-15,17,29H2,1-11H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Retrophin, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at alpha7 nAChR (unknown origin) |
Bioorg Med Chem Lett 26: 3010-3 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.022
BindingDB Entry DOI: 10.7270/Q2R49SPQ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50528722
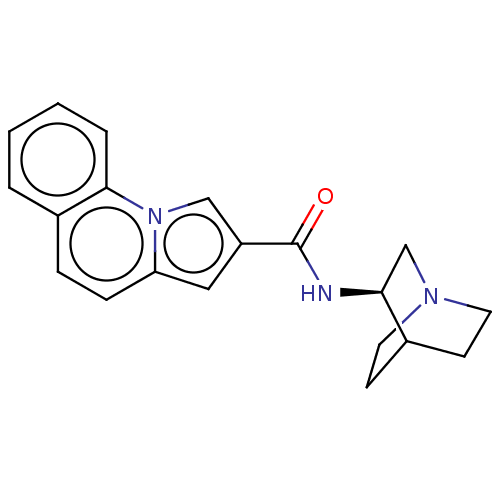
(CHEMBL4453847)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccc3ccccc3n2c1 |r,wD:3.2,(16.19,-4.96,;15.4,-6.28,;16.16,-7.62,;17.7,-7.64,;18.44,-8.99,;19.98,-9.02,;20.77,-7.7,;20.02,-6.36,;18.48,-6.33,;18.69,-7.65,;19.74,-7.81,;13.87,-6.26,;12.98,-5,;11.5,-5.47,;10.17,-4.68,;8.83,-5.45,;8.83,-6.98,;7.49,-7.74,;7.48,-9.27,;8.81,-10.06,;10.15,-9.3,;10.16,-7.76,;11.49,-7.01,;12.95,-7.49,)| Show InChI InChI=1S/C20H21N3O/c24-20(21-18-13-22-9-7-14(18)8-10-22)16-11-17-6-5-15-3-1-2-4-19(15)23(17)12-16/h1-6,11-12,14,18H,7-10,13H2,(H,21,24)/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Induction of desensitization at human alpha7 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine induced current treate... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111618
BindingDB Entry DOI: 10.7270/Q2MG7SZR |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50590141

(CHEMBL5176133)Show SMILES CCCC[C@@H]1N[C@@H](CO)C(=O)NCC(=O)N[C@H]2CSSC[C@@H]3NC(=O)[C@@H](NC(=O)[C@H](Cc4ccc(O)cc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@@H]4CSSC[C@H](NC(=O)[C@@H]5CCCN5C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H]5CCCN5C(=O)CN)C(=O)N[C@@H](CO)C(=O)N4)NC(=O)[C@@H](NC3=O)[C@@H](C)O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)N[C@@H](CCCC)C(=O)NC2=O)[C@@H](C)O)C(O)=O)C(=O)NC1=O)C(C)C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00188h
BindingDB Entry DOI: 10.7270/Q2FF3X9G |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50466606
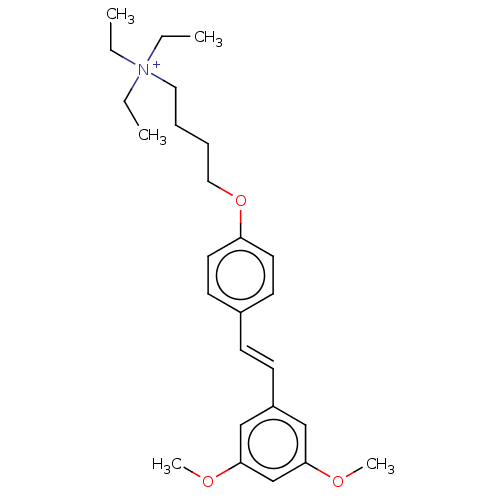
(CHEMBL4280452)Show SMILES [I-].CC[N+](CC)(CC)CCCCOc1ccc(\C=C\c2cc(OC)cc(OC)c2)cc1 Show InChI InChI=1S/C26H38NO3/c1-6-27(7-2,8-3)17-9-10-18-30-24-15-13-22(14-16-24)11-12-23-19-25(28-4)21-26(20-23)29-5/h11-16,19-21H,6-10,17-18H2,1-5H3/q+1/b12-11+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha7 nACHR expressed in xenopous laevis oocyte assessed as inhibition of ACh-induced channel current after 5 mins at -... |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50170588
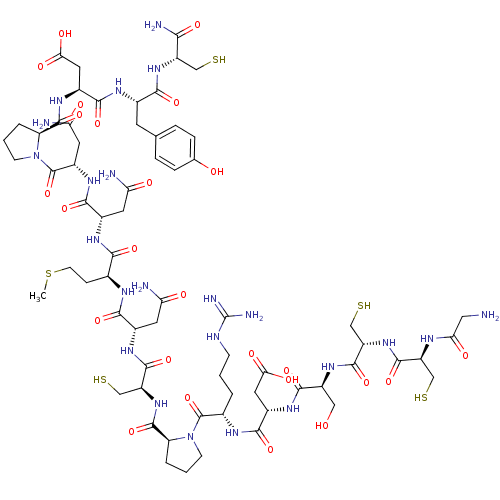
(CHEMBL451252)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C67H103N23O25S5/c1-120-16-12-31(53(102)80-35(19-47(70)94)56(105)84-38(20-48(71)95)66(115)90-15-4-6-44(90)63(112)83-37(22-51(99)100)58(107)79-33(17-29-8-10-30(92)11-9-29)54(103)86-40(25-116)52(72)101)77-55(104)34(18-46(69)93)81-61(110)43(28-119)88-64(113)45-7-3-14-89(45)65(114)32(5-2-13-75-67(73)74)78-57(106)36(21-50(97)98)82-59(108)39(24-91)85-62(111)42(27-118)87-60(109)41(26-117)76-49(96)23-68/h8-11,31-45,91-92,116-119H,2-7,12-28,68H2,1H3,(H2,69,93)(H2,70,94)(H2,71,95)(H2,72,101)(H,76,96)(H,77,104)(H,78,106)(H,79,107)(H,80,102)(H,81,110)(H,82,108)(H,83,112)(H,84,105)(H,85,111)(H,86,103)(H,87,109)(H,88,113)(H,97,98)(H,99,100)(H4,73,74,75)/t31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha 7 |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of [3H]alpha-BGT binding to alpha7 nicotinic acetylcholine receptor in Homo sapiens (human) SH-SY5Y cells |
J Agric Food Chem 48: 6016-24 (2000)
Article DOI: 10.1021/jf000873c
BindingDB Entry DOI: 10.7270/Q27H1NGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM80642

(CHEMBL191491 | MG 624 | MLS002172460 | SMR00125409...)Show InChI InChI=1S/C22H30NO/c1-4-23(5-2,6-3)18-19-24-22-16-14-21(15-17-22)13-12-20-10-8-7-9-11-20/h7-17H,4-6,18-19H2,1-3H3/q+1/b13-12+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha7 nACHR expressed in xenopous laevis oocyte assessed as inhibition of ACh-induced channel current after 5 mins at -... |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50466613

(CHEMBL4287656)Show SMILES [I-].CC[N+](CC)(CC)CCOc1ccc(\C=C\c2cc(OC)cc(OC)c2)cc1 Show InChI InChI=1S/C24H34NO3/c1-6-25(7-2,8-3)15-16-28-22-13-11-20(12-14-22)9-10-21-17-23(26-4)19-24(18-21)27-5/h9-14,17-19H,6-8,15-16H2,1-5H3/q+1/b10-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha7 nACHR expressed in xenopous laevis oocyte assessed as inhibition of ACh-induced channel current after 5 mins at -... |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50170603
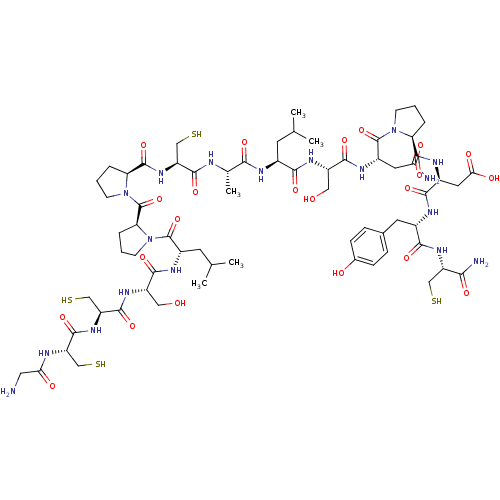
((3S)-3-{[(2S)-1-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C67H104N18O22S4/c1-31(2)19-36(55(95)78-41(25-86)59(99)77-40(22-50(69)89)66(106)83-16-6-9-47(83)63(103)75-38(23-52(91)92)57(97)74-37(21-34-12-14-35(88)15-13-34)56(96)80-43(27-108)53(70)93)73-54(94)33(5)71-60(100)45(29-110)82-64(104)48-10-7-17-84(48)67(107)49-11-8-18-85(49)65(105)39(20-32(3)4)76-58(98)42(26-87)79-62(102)46(30-111)81-61(101)44(28-109)72-51(90)24-68/h12-15,31-33,36-49,86-88,108-111H,6-11,16-30,68H2,1-5H3,(H2,69,89)(H2,70,93)(H,71,100)(H,72,90)(H,73,94)(H,74,97)(H,75,103)(H,76,98)(H,77,99)(H,78,95)(H,79,102)(H,80,96)(H,81,101)(H,82,104)(H,91,92)/t33-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha 7 |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50514670

(CHEMBL4555265)Show SMILES [H][C@]12CSSC[C@H](NC(=O)CNC(=O)CCOCCOCCOCCOCCOCCOCCOCCOCCOCCn3cc(CCCC(=O)NCCCCC(NC(=O)CCCc4cn(CCOCCOCCOCCOCCOCCOCCOCCOCCOCCC(=O)NCC(=O)N[C@H]5CSSC[C@]6([H])NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]7CCCN7C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@]([H])(CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc7ccc(O)cc7)NC(=O)[C@H](CCCNC(N)=N)NC6=O)C(N)=O)NC5=O)nn4)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O)nn3)C(=O)N[C@@]([H])(CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| Show InChI InChI=1S/C194H327N71O61S8/c195-148(270)99-231-161(285)122(20-5-45-219-185(198)199)237-163(287)124(22-7-47-221-187(202)203)239-164(288)125(23-8-48-222-188(204)205)238-162(286)123(21-6-46-220-186(200)201)234-155(277)102-232-160(284)121(233-152(274)33-4-18-118-104-263(261-259-118)58-62-312-66-70-316-74-78-320-82-86-324-90-94-326-92-88-322-84-80-318-76-72-314-68-64-310-60-43-151(273)230-101-154(276)236-141-110-330-334-112-143-178(302)243-127(25-10-50-224-190(208)209)166(290)247-133(96-116-36-40-120(269)41-37-116)172(296)241-129(27-12-52-226-192(212)213)168(292)253-139(159(197)283)108-328-332-114-145(257-176(141)300)180(304)251-137(106-267)174(298)249-135(98-157(280)281)184(308)265-56-16-31-147(265)182(306)245-131(170(294)255-143)29-14-54-228-194(216)217)19-1-2-44-218-149(271)32-3-17-117-103-262(260-258-117)57-61-311-65-69-315-73-77-319-81-85-323-89-93-325-91-87-321-83-79-317-75-71-313-67-63-309-59-42-150(272)229-100-153(275)235-140-109-329-333-111-142-177(301)242-126(24-9-49-223-189(206)207)165(289)246-132(95-115-34-38-119(268)39-35-115)171(295)240-128(26-11-51-225-191(210)211)167(291)252-138(158(196)282)107-327-331-113-144(256-175(140)299)179(303)250-136(105-266)173(297)248-134(97-156(278)279)183(307)264-55-15-30-146(264)181(305)244-130(169(293)254-142)28-13-53-227-193(214)215/h34-41,103-104,121-147,266-269H,1-33,42-102,105-114H2,(H2,195,270)(H2,196,282)(H2,197,283)(H,218,271)(H,229,272)(H,230,273)(H,231,285)(H,232,284)(H,233,274)(H,234,277)(H,235,275)(H,236,276)(H,237,287)(H,238,286)(H,239,288)(H,240,295)(H,241,296)(H,242,301)(H,243,302)(H,244,305)(H,245,306)(H,246,289)(H,247,290)(H,248,297)(H,249,298)(H,250,303)(H,251,304)(H,252,291)(H,253,292)(H,254,293)(H,255,294)(H,256,299)(H,257,300)(H,278,279)(H,280,281)(H4,198,199,219)(H4,200,201,220)(H4,202,203,221)(H4,204,205,222)(H4,206,207,223)(H4,208,209,224)(H4,210,211,225)(H4,212,213,226)(H4,214,215,227)(H4,216,217,228)/t121?,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,142-,143-,144-,145-,146-,147-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Ocean University of China
Curated by ChEMBL
| Assay Description
Inhibition of human alpha7 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of ACh-evoked currents by two-electrode voltage clamp ass... |
J Med Chem 63: 2974-2985 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01536
BindingDB Entry DOI: 10.7270/Q2Z89GSK |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50597510
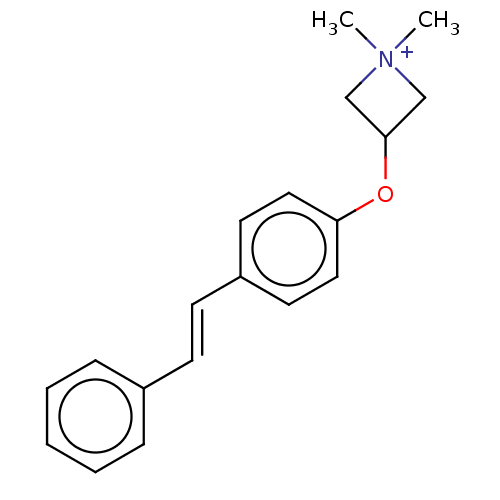
(CHEMBL5176690)Show SMILES [I-].C[N+]1(C)CC(C1)Oc1ccc(\C=C\c2ccccc2)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00746
BindingDB Entry DOI: 10.7270/Q2J96BDG |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50587094
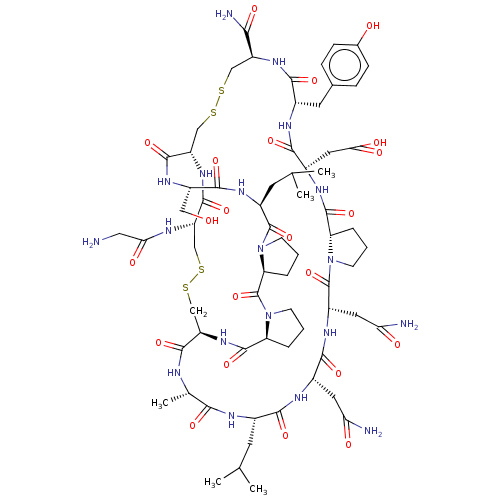
(CHEMBL5083247)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](C)NC(=O)[C@H]2CSSC[C@H](NC(=O)CN)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](Cc3ccc(O)cc3)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]3CCCN3C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC1=O)C(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N2 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human alpha7 nAChR expressed in xenopus oocytes assessed as inhibition of Ach- induced response at -70 mV holding potential pr... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02079
BindingDB Entry DOI: 10.7270/Q2R78K4Q |
More data for this
Ligand-Target Pair | |
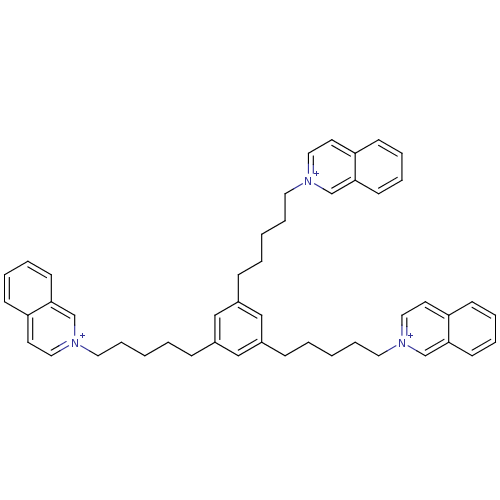
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50339936
(2,2',2''-(5,5',5''-(benzene-1,3,5-triyl)tris(penta...)Show SMILES C(CCc1cc(CCCCC[n+]2ccc3ccccc3c2)cc(CCCCC[n+]2ccc3ccccc3c2)c1)CC[n+]1ccc2ccccc2c1 Show InChI InChI=1S/C48H54N3/c1(13-28-49-31-25-43-19-7-10-22-46(43)37-49)4-16-40-34-41(17-5-2-14-29-50-32-26-44-20-8-11-23-47(44)38-50)36-42(35-40)18-6-3-15-30-51-33-27-45-21-9-12-24-48(45)39-51/h7-12,19-27,31-39H,1-6,13-18,28-30H2/q+3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha7 nAChR expressed in Xenopus oocyte assessed as inhibition of ACh-gated current by voltage clamp electrophysiology assay |
Bioorg Med Chem Lett 21: 2476-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.043
BindingDB Entry DOI: 10.7270/Q2TD9XN9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50466608

(CHEMBL4285772)Show SMILES [I-].CC[N+](CC)(CC)CCCCCCCCOc1ccc(\C=C\c2ccccc2)cc1 Show InChI InChI=1S/C28H42NO/c1-4-29(5-2,6-3)24-14-9-7-8-10-15-25-30-28-22-20-27(21-23-28)19-18-26-16-12-11-13-17-26/h11-13,16-23H,4-10,14-15,24-25H2,1-3H3/q+1/b19-18+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha7 nACHR expressed in xenopous laevis oocyte assessed as inhibition of ACh-induced channel current after 5 mins at -... |
J Med Chem 61: 10531-10544 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01052
BindingDB Entry DOI: 10.7270/Q2PV6P2W |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50339938
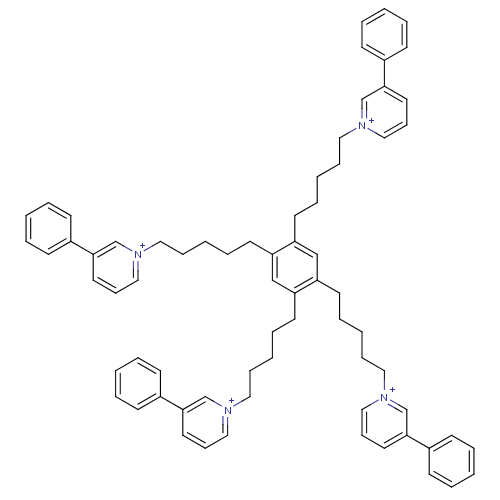
(1,1',1'',1'''-(5,5',5'',5'''-(benzene-1,2,4,5-tetr...)Show SMILES C(CCc1cc(CCCCC[n+]2cccc(c2)-c2ccccc2)c(CCCCC[n+]2cccc(c2)-c2ccccc2)cc1CCCCC[n+]1cccc(c1)-c1ccccc1)CC[n+]1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C70H78N4/c1-9-29-59(30-10-1)67-41-25-49-71(55-67)45-21-5-17-37-63-53-65(39-19-7-23-47-73-51-27-43-69(57-73)61-33-13-3-14-34-61)66(40-20-8-24-48-74-52-28-44-70(58-74)62-35-15-4-16-36-62)54-64(63)38-18-6-22-46-72-50-26-42-68(56-72)60-31-11-2-12-32-60/h1-4,9-16,25-36,41-44,49-58H,5-8,17-24,37-40,45-48H2/q+4 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha7 nAChR expressed in Xenopus oocyte assessed as inhibition of ACh-gated current by voltage clamp electrophysiology assay |
Bioorg Med Chem Lett 21: 2476-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.043
BindingDB Entry DOI: 10.7270/Q2TD9XN9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50445327
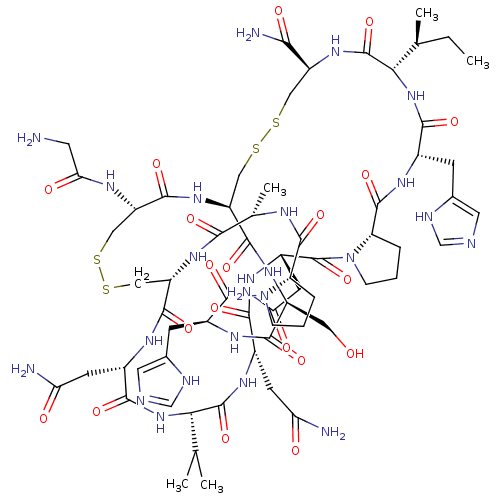
(CHEMBL3104240)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H]2CSSC[C@H](NC(=O)CN)C(=O)N[C@@H](CSSC[C@H](NC1=O)C(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N2)C(C)C |r| Show InChI InChI=1S/C65H98N24O20S4/c1-6-29(4)50-63(107)83-39(51(70)95)22-110-112-25-42-59(103)82-38(21-90)56(100)80-36(14-32-20-72-27-74-32)64(108)88-11-7-9-43(88)60(104)75-30(5)52(96)84-41(24-113-111-23-40(57(101)85-42)76-48(94)18-66)58(102)77-35(16-46(68)92)55(99)86-49(28(2)3)62(106)79-34(15-45(67)91)53(97)81-37(17-47(69)93)65(109)89-12-8-10-44(89)61(105)78-33(54(98)87-50)13-31-19-71-26-73-31/h19-20,26-30,33-44,49-50,90H,6-18,21-25,66H2,1-5H3,(H2,67,91)(H2,68,92)(H2,69,93)(H2,70,95)(H,71,73)(H,72,74)(H,75,104)(H,76,94)(H,77,102)(H,78,105)(H,79,106)(H,80,100)(H,81,97)(H,82,103)(H,83,107)(H,84,96)(H,85,101)(H,86,99)(H,87,98)/t29-,30-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,49-,50-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Hainan University
Curated by ChEMBL
| Assay Description
Inhibition of alpha7 nAChR (unknown origin) expressed in Xenopus laevis oocytes by two-electrode voltage clamp method |
J Med Chem 56: 9655-63 (2014)
Article DOI: 10.1021/jm401254c
BindingDB Entry DOI: 10.7270/Q22Z171M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM80642

(CHEMBL191491 | MG 624 | MLS002172460 | SMR00125409...)Show InChI InChI=1S/C22H30NO/c1-4-23(5-2,6-3)18-19-24-22-16-14-21(15-17-22)13-12-20-10-8-7-9-11-20/h7-17H,4-6,18-19H2,1-3H3/q+1/b13-12+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha 7 |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50606633
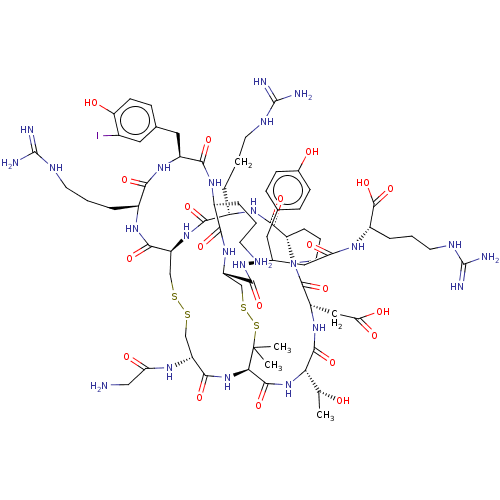
(CHEMBL5219936)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H]1NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2ccc(O)c(I)c2)C(=O)N[C@@H](CCCN)C(=O)N[C@@H](CSSC1(C)C)C(=O)N[C@H](CC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)Cc1ccc(O)cc1)NC(=O)CN)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00512
BindingDB Entry DOI: 10.7270/Q20G3Q8V |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50339935

(1,1',1''-(5,5',5''-(benzene-1,3,5-triyl)tris(penta...)Show SMILES C(CCc1cc(CCCCC[n+]2cccc3ccccc23)cc(CCCCC[n+]2cccc3ccccc23)c1)CC[n+]1cccc2ccccc12 Show InChI InChI=1S/C48H54N3/c1(13-31-49-34-16-25-43-22-7-10-28-46(43)49)4-19-40-37-41(20-5-2-14-32-50-35-17-26-44-23-8-11-29-47(44)50)39-42(38-40)21-6-3-15-33-51-36-18-27-45-24-9-12-30-48(45)51/h7-12,16-18,22-30,34-39H,1-6,13-15,19-21,31-33H2/q+3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha7 nAChR expressed in Xenopus oocyte assessed as inhibition of ACh-gated current by voltage clamp electrophysiology assay |
Bioorg Med Chem Lett 21: 2476-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.043
BindingDB Entry DOI: 10.7270/Q2TD9XN9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50590140

(CHEMBL5202162)Show SMILES CCCC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)[C@@H](C)O)C(=O)N[C@H]1CSSC[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCC)NC(=O)[C@H](CO)NC(=O)CNC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N3)NC(=O)CNC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC2=O)C(C)C)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O)[C@@H](C)O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00188h
BindingDB Entry DOI: 10.7270/Q2FF3X9G |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50339933
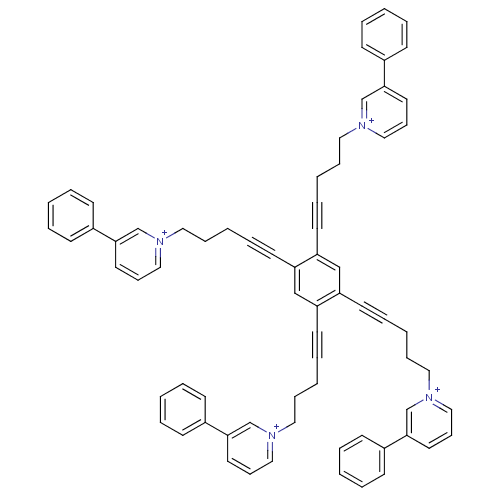
(1,1',1'',1'''-(5,5',5'',5'''-(benzene-1,2,4,5-tetr...)Show SMILES C(CC#Cc1cc(C#CCCC[n+]2cccc(c2)-c2ccccc2)c(cc1C#CCCC[n+]1cccc(c1)-c1ccccc1)C#CCCC[n+]1cccc(c1)-c1ccccc1)C[n+]1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C70H62N4/c1-9-29-59(30-10-1)67-41-25-49-71(55-67)45-21-5-17-37-63-53-65(39-19-7-23-47-73-51-27-43-69(57-73)61-33-13-3-14-34-61)66(40-20-8-24-48-74-52-28-44-70(58-74)62-35-15-4-16-36-62)54-64(63)38-18-6-22-46-72-50-26-42-68(56-72)60-31-11-2-12-32-60/h1-4,9-16,25-36,41-44,49-58H,5-8,21-24,45-48H2/q+4 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha7 nAChR expressed in Xenopus oocyte assessed as inhibition of ACh-gated current by voltage clamp electrophysiology assay |
Bioorg Med Chem Lett 21: 2476-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.043
BindingDB Entry DOI: 10.7270/Q2TD9XN9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50581246

(CHEMBL5080428)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H]2NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]3CCCN3C(=O)[C@H](CC(O)=O)NC1=O)C(=O)N[C@@H](CCCNC(N)=O)C(=O)N[C@@H](Cc1ccc(O)c(I)c1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CSCS2)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O)NC(=O)CN |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human alpha7 nAChR expressed in Xenopus laevis oocytes assessed as inhibition of acetylcholine-induced current response by two-electrod... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00802
BindingDB Entry DOI: 10.7270/Q2542SG3 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50170593
((3S)-3-[(2S)-2-[(2R)-2-[(2R)-2-(2-aminoacetamido)-...)Show SMILES C[C@H](NC(=O)[C@H](CS)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)CN)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O Show InChI InChI=1S/C49H77N19O14S3/c1-23(39(74)63-29(15-24-18-58-26-8-3-2-7-25(24)26)41(76)61-27(38(51)73)9-4-12-56-48(52)53)59-43(78)33(21-84)66-40(75)28(10-5-13-57-49(54)55)62-46(81)35-11-6-14-68(35)47(82)30(16-37(71)72)64-42(77)31(19-69)65-45(80)34(22-85)67-44(79)32(20-83)60-36(70)17-50/h2-3,7-8,18,23,27-35,58,69,83-85H,4-6,9-17,19-22,50H2,1H3,(H2,51,73)(H,59,78)(H,60,70)(H,61,76)(H,62,81)(H,63,74)(H,64,77)(H,65,80)(H,66,75)(H,67,79)(H,71,72)(H4,52,53,56)(H4,54,55,57)/t23-,27-,28-,29-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Nicotinic acetylcholine receptor alpha7; Range is 100-200 nM |
J Med Chem 48: 4705-45 (2005)
Article DOI: 10.1021/jm040219e
BindingDB Entry DOI: 10.7270/Q29W0G79 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50485643
(3-Phenylquinuclidine | CHEMBL1890808)Show SMILES C1CN2CCC1C(C2)c1ccccc1 |THB:8:6:0.1:4.3,(-1.15,-3.06,;-.95,-4.47,;.61,-3.8,;.87,-1.86,;.42,-.73,;.35,-2.4,;1.72,-3.02,;2.01,-4.45,;2.93,-2.06,;2.7,-.54,;3.9,.42,;5.34,-.15,;5.57,-1.67,;4.36,-2.63,)| Show InChI InChI=1S/C13H17N/c1-2-4-11(5-3-1)13-10-14-8-6-12(13)7-9-14/h1-5,12-13H,6-10H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bezmialem Vakif University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from chimeric human alpha7-5-HT3A receptor expressed in HEK293 cells after 2 hrs by scintillation counter analysis |
Bioorg Med Chem 20: 5992-6002 (2012)
Article DOI: 10.1016/j.bmc.2012.06.054
BindingDB Entry DOI: 10.7270/Q2G73HMS |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50601896

(CHEMBL5203781)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@@H]2CSSC[C@H](NC(=O)CN)C(=O)N[C@@H](CSSC[C@H](NC1=O)C(N)=O)C(=O)NC(CN)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N2)C(C)C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 259 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00494
BindingDB Entry DOI: 10.7270/Q2SB49T6 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50601896

(CHEMBL5203781)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@@H]2CSSC[C@H](NC(=O)CN)C(=O)N[C@@H](CSSC[C@H](NC1=O)C(N)=O)C(=O)NC(CN)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N2)C(C)C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 266 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00494
BindingDB Entry DOI: 10.7270/Q2SB49T6 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50220056
((1S,9R,18R,19S,21R,22R,23R,25R,32S,34R,35R,36S)-24...)Show SMILES C=CC[N+]12CC[C@H]3[C@@H]1C[C@@H]1[C@@H]4[C@@H]3N([C@@H]3OCC=C5C[N+]6(CC=C)CC[C@@]78[C@@H]6C[C@@H]5[C@@H]3[C@@H]7N([C@@H]4OCC=C1C2)c1ccccc81)c1ccccc1 |w:3.2,19.21,c:42,t:18,TEB:20:19:25.30.29:27,2:3:8:11.10.6| Show InChI InChI=1S/C44H52N4O2/c1-3-18-47-20-14-31-36(47)24-32-28(26-47)15-22-49-42-38(32)40(31)45(30-10-6-5-7-11-30)43-39-33-25-37-44(34-12-8-9-13-35(34)46(42)41(39)44)17-21-48(37,19-4-2)27-29(33)16-23-50-43/h3-13,15-16,31-33,36-43H,1-2,14,17-27H2/q+2/t31-,32-,33-,36-,37-,38+,39+,40+,41-,42+,43+,44+,47?,48?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Antagonist activity at human alpha-7 nAChR in tsA201 cells coexpressed with Ric3 by FMP assay |
J Med Chem 50: 4616-29 (2007)
Article DOI: 10.1021/jm070574f
BindingDB Entry DOI: 10.7270/Q2J67HRC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50459888
(CHEMBL4225157)Show SMILES C#CCNc1nc(NCCCCC2CCN(Cc3ccccc3)CC2)c(cc1C#N)C#N Show InChI InChI=1S/C26H30N6/c1-2-13-29-25-23(18-27)17-24(19-28)26(31-25)30-14-7-6-8-21-11-15-32(16-12-21)20-22-9-4-3-5-10-22/h1,3-5,9-10,17,21H,6-8,11-16,20H2,(H2,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human alpha7 nAChR expressed in Xenopus oocytes at -80 mV holding potential by electro-physiological analysis |
Bioorg Med Chem Lett 27: 3207-3218 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.073
BindingDB Entry DOI: 10.7270/Q2MC92NR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data