 Found 21 hits of ki for UniProtKB: P06869
Found 21 hits of ki for UniProtKB: P06869 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50531924
(CHEMBL4464577)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](N)CSSC[C@H](NC(=O)CNC1=O)C(O)=O |r| Show InChI InChI=1S/C49H71N13O14S2/c1-4-25(2)39-46(73)54-21-38(66)56-36(48(75)76)24-78-77-23-31(50)47(74)62-18-6-8-37(62)45(72)55-26(3)40(67)58-33(19-27-9-13-29(64)14-10-27)42(69)60-35(22-63)44(71)57-32(7-5-17-53-49(51)52)41(68)59-34(43(70)61-39)20-28-11-15-30(65)16-12-28/h9-16,25-26,31-37,39,63-65H,4-8,17-24,50H2,1-3H3,(H,54,73)(H,55,72)(H,56,66)(H,57,71)(H,58,67)(H,59,68)(H,60,69)(H,61,70)(H,75,76)(H4,51,52,53)/t25-,26-,31-,32-,33-,34-,35-,36-,37-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA preincubated for 15 mins followed by chromogenic substrate addition |
J Med Chem 62: 2172-2183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01908
BindingDB Entry DOI: 10.7270/Q24F1V6R |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50531924

(CHEMBL4464577)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](N)CSSC[C@H](NC(=O)CNC1=O)C(O)=O |r| Show InChI InChI=1S/C49H71N13O14S2/c1-4-25(2)39-46(73)54-21-38(66)56-36(48(75)76)24-78-77-23-31(50)47(74)62-18-6-8-37(62)45(72)55-26(3)40(67)58-33(19-27-9-13-29(64)14-10-27)42(69)60-35(22-63)44(71)57-32(7-5-17-53-49(51)52)41(68)59-34(43(70)61-39)20-28-11-15-30(65)16-12-28/h9-16,25-26,31-37,39,63-65H,4-8,17-24,50H2,1-3H3,(H,54,73)(H,55,72)(H,56,66)(H,57,71)(H,58,67)(H,59,68)(H,60,69)(H,61,70)(H,75,76)(H4,51,52,53)/t25-,26-,31-,32-,33-,34-,35-,36-,37-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA serine protease domain preincubated for 15 mins followed by chromogenic substrate addition |
J Med Chem 62: 2172-2183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01908
BindingDB Entry DOI: 10.7270/Q24F1V6R |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50531926

(CHEMBL4550668)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CSSC[C@H](NC(=O)CNC1=O)C(N)=O)NC(C)=O |r| Show InChI InChI=1S/C51H74N14O14S2/c1-5-26(2)41-49(78)56-22-40(70)59-37(42(52)71)24-80-81-25-38(58-28(4)67)50(79)65-19-7-9-39(65)48(77)57-27(3)43(72)61-34(20-29-10-14-31(68)15-11-29)45(74)63-36(23-66)47(76)60-33(8-6-18-55-51(53)54)44(73)62-35(46(75)64-41)21-30-12-16-32(69)17-13-30/h10-17,26-27,33-39,41,66,68-69H,5-9,18-25H2,1-4H3,(H2,52,71)(H,56,78)(H,57,77)(H,58,67)(H,59,70)(H,60,76)(H,61,72)(H,62,73)(H,63,74)(H,64,75)(H4,53,54,55)/t26-,27-,33-,34-,35-,36-,37-,38-,39-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA preincubated for 15 mins followed by chromogenic substrate addition |
J Med Chem 62: 2172-2183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01908
BindingDB Entry DOI: 10.7270/Q24F1V6R |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50531926

(CHEMBL4550668)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CSSC[C@H](NC(=O)CNC1=O)C(N)=O)NC(C)=O |r| Show InChI InChI=1S/C51H74N14O14S2/c1-5-26(2)41-49(78)56-22-40(70)59-37(42(52)71)24-80-81-25-38(58-28(4)67)50(79)65-19-7-9-39(65)48(77)57-27(3)43(72)61-34(20-29-10-14-31(68)15-11-29)45(74)63-36(23-66)47(76)60-33(8-6-18-55-51(53)54)44(73)62-35(46(75)64-41)21-30-12-16-32(69)17-13-30/h10-17,26-27,33-39,41,66,68-69H,5-9,18-25H2,1-4H3,(H2,52,71)(H,56,78)(H,57,77)(H,58,67)(H,59,70)(H,60,76)(H,61,72)(H,62,73)(H,63,74)(H,64,75)(H4,53,54,55)/t26-,27-,33-,34-,35-,36-,37-,38-,39-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA |
J Med Chem 62: 2172-2183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01908
BindingDB Entry DOI: 10.7270/Q24F1V6R |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50499240

(CHEMBL3735080)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C54H77N13O16S2/c1-27(2)19-35-45(74)63-39(23-43(71)72)49(78)65-41(53(82)83)26-85-84-25-34(55)52(81)67-16-4-5-42(67)51(80)58-28(3)44(73)59-36(20-29-6-10-32(69)11-7-29)48(77)64-40(24-68)50(79)62-38(22-31-14-17-66(18-15-31)54(56)57)47(76)61-37(46(75)60-35)21-30-8-12-33(70)13-9-30/h6-13,27-28,31,34-42,68-70H,4-5,14-26,55H2,1-3H3,(H3,56,57)(H,58,80)(H,59,73)(H,60,75)(H,61,76)(H,62,79)(H,63,74)(H,64,77)(H,65,78)(H,71,72)(H,82,83)/t28-,34-,35-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA for 15 mins using pyro-Glu-Gly-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50531926

(CHEMBL4550668)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CSSC[C@H](NC(=O)CNC1=O)C(N)=O)NC(C)=O |r| Show InChI InChI=1S/C51H74N14O14S2/c1-5-26(2)41-49(78)56-22-40(70)59-37(42(52)71)24-80-81-25-38(58-28(4)67)50(79)65-19-7-9-39(65)48(77)57-27(3)43(72)61-34(20-29-10-14-31(68)15-11-29)45(74)63-36(23-66)47(76)60-33(8-6-18-55-51(53)54)44(73)62-35(46(75)64-41)21-30-12-16-32(69)17-13-30/h10-17,26-27,33-39,41,66,68-69H,5-9,18-25H2,1-4H3,(H2,52,71)(H,56,78)(H,57,77)(H,58,67)(H,59,70)(H,60,76)(H,61,72)(H,62,73)(H,63,74)(H,64,75)(H4,53,54,55)/t26-,27-,33-,34-,35-,36-,37-,38-,39-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA serine protease domain preincubated for 15 mins followed by chromogenic substrate addition |
J Med Chem 62: 2172-2183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01908
BindingDB Entry DOI: 10.7270/Q24F1V6R |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50531925

(CHEMBL4522772)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC1=O)C(N)=O)NC(C)=O |r| Show InChI InChI=1S/C53H76N14O16S2/c1-26(2)19-34-46(77)64-37(22-42(72)73)49(80)66-39(43(54)74)24-84-85-25-40(59-28(4)69)52(83)67-18-6-8-41(67)51(82)58-27(3)44(75)61-35(20-29-9-13-31(70)14-10-29)48(79)65-38(23-68)50(81)60-33(7-5-17-57-53(55)56)45(76)63-36(47(78)62-34)21-30-11-15-32(71)16-12-30/h9-16,26-27,33-41,68,70-71H,5-8,17-25H2,1-4H3,(H2,54,74)(H,58,82)(H,59,69)(H,60,81)(H,61,75)(H,62,78)(H,63,76)(H,64,77)(H,65,79)(H,66,80)(H,72,73)(H4,55,56,57)/t27-,33-,34-,35-,36-,37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA preincubated for 15 mins followed by chromogenic substrate addition |
J Med Chem 62: 2172-2183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01908
BindingDB Entry DOI: 10.7270/Q24F1V6R |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50531925

(CHEMBL4522772)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC1=O)C(N)=O)NC(C)=O |r| Show InChI InChI=1S/C53H76N14O16S2/c1-26(2)19-34-46(77)64-37(22-42(72)73)49(80)66-39(43(54)74)24-84-85-25-40(59-28(4)69)52(83)67-18-6-8-41(67)51(82)58-27(3)44(75)61-35(20-29-9-13-31(70)14-10-29)48(79)65-38(23-68)50(81)60-33(7-5-17-57-53(55)56)45(76)63-36(47(78)62-34)21-30-11-15-32(71)16-12-30/h9-16,26-27,33-41,68,70-71H,5-8,17-25H2,1-4H3,(H2,54,74)(H,58,82)(H,59,69)(H,60,81)(H,61,75)(H,62,78)(H,63,76)(H,64,77)(H,65,79)(H,66,80)(H,72,73)(H4,55,56,57)/t27-,33-,34-,35-,36-,37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA |
J Med Chem 62: 2172-2183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01908
BindingDB Entry DOI: 10.7270/Q24F1V6R |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50499239

(CHEMBL3734777)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C51H73N13O16S2/c1-25(2)18-33-43(72)61-36(21-40(68)69)46(75)63-38(50(79)80)24-82-81-23-31(52)49(78)64-17-5-7-39(64)48(77)56-26(3)41(70)58-34(19-27-8-12-29(66)13-9-27)45(74)62-37(22-65)47(76)57-32(6-4-16-55-51(53)54)42(71)60-35(44(73)59-33)20-28-10-14-30(67)15-11-28/h8-15,25-26,31-39,65-67H,4-7,16-24,52H2,1-3H3,(H,56,77)(H,57,76)(H,58,70)(H,59,73)(H,60,71)(H,61,72)(H,62,74)(H,63,75)(H,68,69)(H,79,80)(H4,53,54,55)/t26-,31-,32-,33-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA for 15 mins using pyro-Glu-Gly-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50529264
(CHEMBL4513418)Show InChI InChI=1S/C11H13N9O2/c1-22-11-16-2-4(3-17-11)5-7(12)19-8(13)6(18-5)9(21)20-10(14)15/h2-3H,1H3,(H4,12,13,19)(H4,14,15,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 956 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse uPA using Z-Gly-Gly-Arg-AMC as substrate measured for 15 mins by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01423
BindingDB Entry DOI: 10.7270/Q2639TNC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50459059
(CHEMBL4217043)Show SMILES COc1ncc(cn1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C17H23N9O2/c1-28-17-21-8-10(9-22-17)11-14(26-6-4-2-3-5-7-26)24-13(18)12(23-11)15(27)25-16(19)20/h8-9H,2-7H2,1H3,(H2,18,24)(H4,19,20,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA using Z-Gly-Gly-Arg-AMC as substrate after 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50531925

(CHEMBL4522772)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC1=O)C(N)=O)NC(C)=O |r| Show InChI InChI=1S/C53H76N14O16S2/c1-26(2)19-34-46(77)64-37(22-42(72)73)49(80)66-39(43(54)74)24-84-85-25-40(59-28(4)69)52(83)67-18-6-8-41(67)51(82)58-27(3)44(75)61-35(20-29-9-13-31(70)14-10-29)48(79)65-38(23-68)50(81)60-33(7-5-17-57-53(55)56)45(76)63-36(47(78)62-34)21-30-11-15-32(71)16-12-30/h9-16,26-27,33-41,68,70-71H,5-8,17-25H2,1-4H3,(H2,54,74)(H,58,82)(H,59,69)(H,60,81)(H,61,75)(H,62,78)(H,63,76)(H,64,77)(H,65,79)(H,66,80)(H,72,73)(H4,55,56,57)/t27-,33-,34-,35-,36-,37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA serine protease domain preincubated for 15 mins followed by chromogenic substrate addition |
J Med Chem 62: 2172-2183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01908
BindingDB Entry DOI: 10.7270/Q24F1V6R |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50459054

(CHEMBL4213248)Show SMILES NC(=N)NC(=O)c1nc(-c2cc3ccccc3o2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C20H23N7O2/c21-17-16(19(28)26-20(22)23)24-15(14-11-12-7-3-4-8-13(12)29-14)18(25-17)27-9-5-1-2-6-10-27/h3-4,7-8,11H,1-2,5-6,9-10H2,(H2,21,25)(H4,22,23,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA using Z-Gly-Gly-Arg-AMC as substrate after 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM16173
(3,5-diamino-6-chloro-N-(diaminomethylene)pyrazinam...)Show InChI InChI=1S/C6H8ClN7O/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11/h(H4,8,9,13)(H4,10,11,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA using Z-Gly-Gly-Arg-AMC as substrate after 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50459080
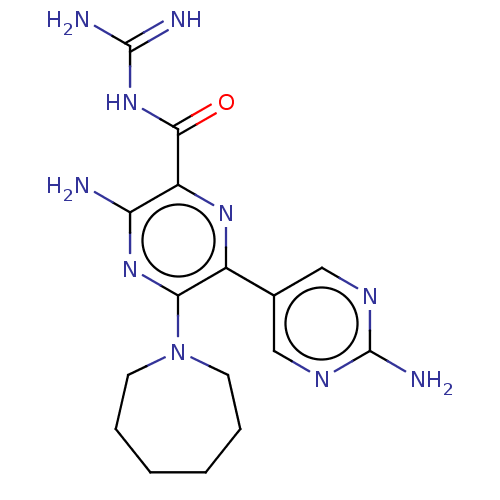
(CHEMBL4211423)Show SMILES NC(=N)NC(=O)c1nc(-c2cnc(N)nc2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C16H22N10O/c17-12-11(14(27)25-15(18)19)23-10(9-7-21-16(20)22-8-9)13(24-12)26-5-3-1-2-4-6-26/h7-8H,1-6H2,(H2,17,24)(H2,20,21,22)(H4,18,19,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse uPA using Z-Gly-Gly-Arg-AMC as substrate measured for 15 mins by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01423
BindingDB Entry DOI: 10.7270/Q2639TNC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50459079
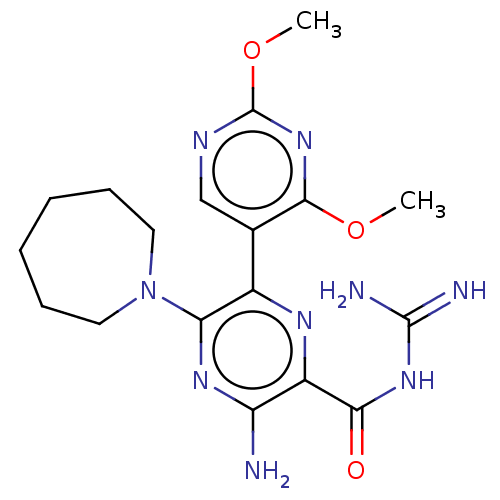
(CHEMBL4206764)Show SMILES COc1ncc(c(OC)n1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C18H25N9O3/c1-29-16-10(9-22-18(26-16)30-2)11-14(27-7-5-3-4-6-8-27)24-13(19)12(23-11)15(28)25-17(20)21/h9H,3-8H2,1-2H3,(H2,19,24)(H4,20,21,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse uPA using Z-Gly-Gly-Arg-AMC as substrate measured for 15 mins by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01423
BindingDB Entry DOI: 10.7270/Q2639TNC |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM81818

(CAS_1794 | CHEMBL1909810 | HMA | NSC_1794)Show InChI InChI=1S/C12H18ClN7O/c13-8-10(20-5-3-1-2-4-6-20)18-9(14)7(17-8)11(21)19-12(15)16/h1-6H2,(H2,14,18)(H4,15,16,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA using Z-Gly-Gly-Arg-AMC as substrate after 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50499238
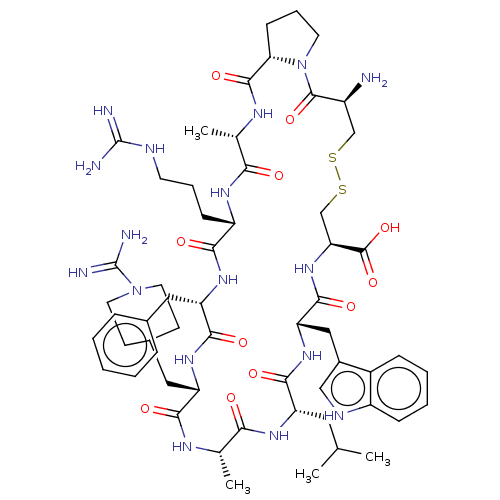
(CHEMBL3735263)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C58H85N17O11S2/c1-31(2)24-41-51(80)72-44(27-36-28-65-39-15-9-8-14-37(36)39)53(82)73-45(56(85)86)30-88-87-29-38(59)55(84)75-21-11-17-46(75)54(83)67-33(4)47(76)68-40(16-10-20-64-57(60)61)49(78)70-43(25-34-12-6-5-7-13-34)52(81)71-42(50(79)66-32(3)48(77)69-41)26-35-18-22-74(23-19-35)58(62)63/h5-9,12-15,28,31-33,35,38,40-46,65H,10-11,16-27,29-30,59H2,1-4H3,(H3,62,63)(H,66,79)(H,67,83)(H,68,76)(H,69,77)(H,70,78)(H,71,81)(H,72,80)(H,73,82)(H,85,86)(H4,60,61,64)/t32-,33-,38-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA for 15 mins using pyro-Glu-Gly-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50499242
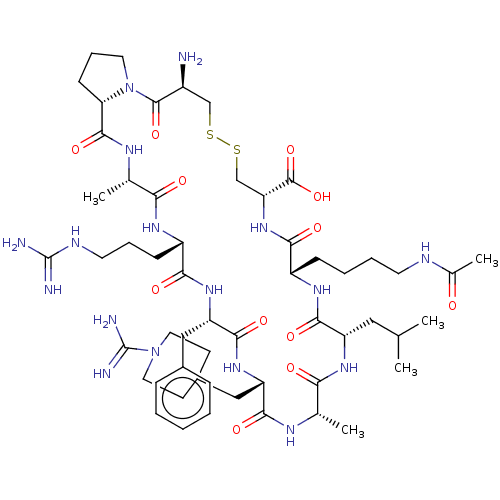
(CHEMBL3736473)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@@H](NC(=O)[C@H](CCCCNC(C)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C55H89N17O12S2/c1-30(2)25-39-49(79)66-37(15-9-10-20-61-33(5)73)47(77)70-42(53(83)84)29-86-85-28-36(56)52(82)72-22-12-17-43(72)51(81)64-32(4)44(74)65-38(16-11-21-62-54(57)58)46(76)68-41(26-34-13-7-6-8-14-34)50(80)69-40(48(78)63-31(3)45(75)67-39)27-35-18-23-71(24-19-35)55(59)60/h6-8,13-14,30-32,35-43H,9-12,15-29,56H2,1-5H3,(H3,59,60)(H,61,73)(H,63,78)(H,64,81)(H,65,74)(H,66,79)(H,67,75)(H,68,76)(H,69,80)(H,70,77)(H,83,84)(H4,57,58,62)/t31-,32-,36-,37-,38-,39-,40-,41-,42+,43-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA for 15 mins using pyro-Glu-Gly-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50499241

(CHEMBL3735513)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC2=O)C(O)=O |r| Show InChI InChI=1S/C56H84N16O11S2/c1-31(2)25-39-49(77)69-42(27-35-15-9-6-10-16-35)51(79)70-43(54(82)83)30-85-84-29-37(57)53(81)72-22-12-18-44(72)52(80)64-33(4)45(73)65-38(17-11-21-62-55(58)59)47(75)67-41(26-34-13-7-5-8-14-34)50(78)68-40(48(76)63-32(3)46(74)66-39)28-36-19-23-71(24-20-36)56(60)61/h5-10,13-16,31-33,36-44H,11-12,17-30,57H2,1-4H3,(H3,60,61)(H,63,76)(H,64,80)(H,65,73)(H,66,74)(H,67,75)(H,68,78)(H,69,77)(H,70,79)(H,82,83)(H4,58,59,62)/t32-,33-,37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA for 15 mins using pyro-Glu-Gly-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50499237

(CHEMBL3735217)Show SMILES [H][C@@]12CCCN1C(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC1CCN(CC1)C(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC2=O)C(O)=O |r| Show InChI InChI=1S/C59H91N17O11S2/c1-34(2)28-42-52(81)73-45(30-37-16-8-5-9-17-37)54(83)74-46(57(86)87)33-89-88-32-39(61)56(85)76-25-13-20-47(76)55(84)69-40(18-10-11-23-60)49(78)68-41(19-12-24-66-58(62)63)50(79)71-44(29-36-14-6-4-7-15-36)53(82)72-43(51(80)67-35(3)48(77)70-42)31-38-21-26-75(27-22-38)59(64)65/h4-9,14-17,34-35,38-47H,10-13,18-33,60-61H2,1-3H3,(H3,64,65)(H,67,80)(H,68,78)(H,69,84)(H,70,77)(H,71,79)(H,72,82)(H,73,81)(H,74,83)(H,86,87)(H4,62,63,66)/t35-,39-,40-,41-,42-,43-,44-,45-,46-,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA for 15 mins using pyro-Glu-Gly-Arg-p-nitroanilide as substrate |
J Med Chem 58: 8868-76 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01128
BindingDB Entry DOI: 10.7270/Q2XK8JH7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data