

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50453918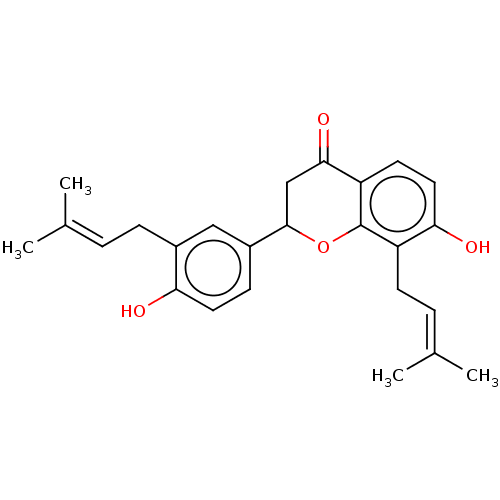 (CHEMBL4078418) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 transfected in HEK293 cells assessed as protection against CYP1A1 mediated B[a]P toxicity by measuring B[a]P EC50 at 20 uM... | Bioorg Med Chem Lett 27: 5400-5403 (2017) Article DOI: 10.1016/j.bmcl.2017.11.013 BindingDB Entry DOI: 10.7270/Q2GF0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 transfected in HEK293 cells assessed as protection against CYP1A1 mediated B[a]P toxicity by measuring B[a]P EC50 at 20 uM... | Bioorg Med Chem Lett 27: 5400-5403 (2017) Article DOI: 10.1016/j.bmcl.2017.11.013 BindingDB Entry DOI: 10.7270/Q2GF0X2H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 transfected in HEK293 cells assessed as protection against CYP1A1 mediated B[a]P toxicity by measuring B[a]P EC50 at 20 uM... | Bioorg Med Chem Lett 27: 5400-5403 (2017) Article DOI: 10.1016/j.bmcl.2017.11.013 BindingDB Entry DOI: 10.7270/Q2GF0X2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50548259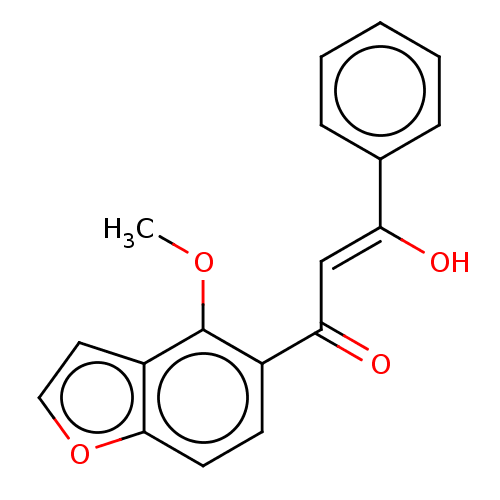 (Pongamol) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CYP1A1 expressed in HEK293 cells assessed as reduction in CYP1A1 mediated B[alpha]P-7,8- dihydrodiol induced-toxicity by measurin... | Citation and Details Article DOI: 10.1016/j.bmc.2018.11.013 BindingDB Entry DOI: 10.7270/Q20V8HDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50452365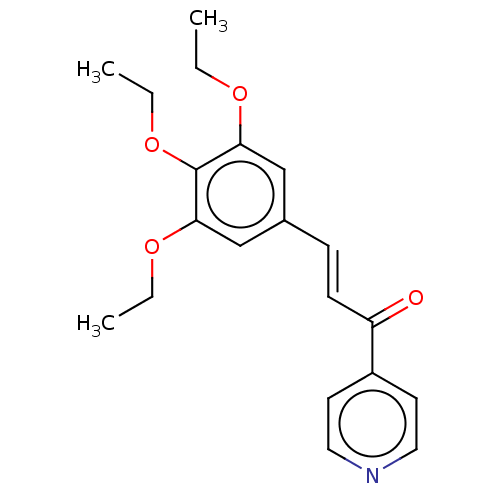 (CHEMBL4211876) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a |
De Montfort University Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in HEK293 cells assessed as reduction in benzo(a)pyrene-induced toxicity by measuring B[a]P EC50 at ... | Bioorg Med Chem Lett 27: 5409-5414 (2017) Article DOI: 10.1016/j.bmcl.2017.11.009 BindingDB Entry DOI: 10.7270/Q2MP55TT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50452359 (CHEMBL4205073) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a |
De Montfort University Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in HEK293 cells assessed as reduction in benzo(a)pyrene-induced toxicity by measuring B[a]P EC50 at ... | Bioorg Med Chem Lett 27: 5409-5414 (2017) Article DOI: 10.1016/j.bmcl.2017.11.009 BindingDB Entry DOI: 10.7270/Q2MP55TT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50452357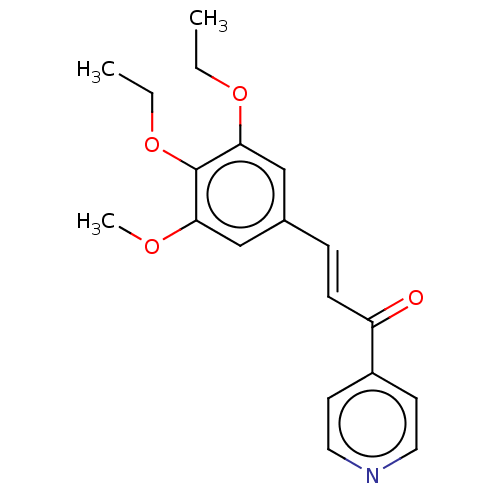 (CHEMBL4210137) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a |
De Montfort University Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in HEK293 cells assessed as reduction in benzo(a)pyrene-induced toxicity by measuring B[a]P EC50 at ... | Bioorg Med Chem Lett 27: 5409-5414 (2017) Article DOI: 10.1016/j.bmcl.2017.11.009 BindingDB Entry DOI: 10.7270/Q2MP55TT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50452361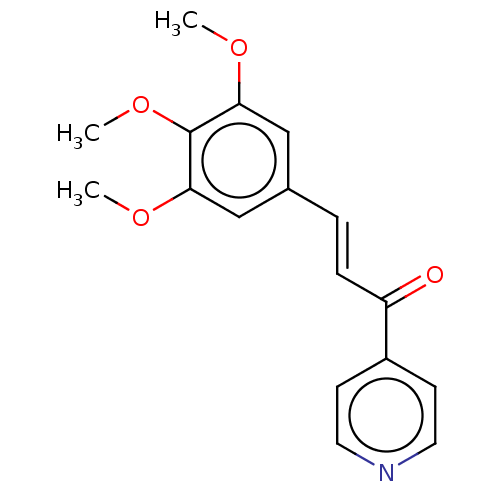 (CHEMBL4218749) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a |
De Montfort University Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in HEK293 cells assessed as reduction in benzo(a)pyrene-induced toxicity by measuring B[a]P EC50 at ... | Bioorg Med Chem Lett 27: 5409-5414 (2017) Article DOI: 10.1016/j.bmcl.2017.11.009 BindingDB Entry DOI: 10.7270/Q2MP55TT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50548258 (LANCEOLATIN B) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CYP1A1 expressed in HEK293 cells assessed as reduction in CYP1A1 mediated B[alpha]P-7,8- dihydrodiol induced-toxicity by measurin... | Citation and Details Article DOI: 10.1016/j.bmc.2018.11.013 BindingDB Entry DOI: 10.7270/Q20V8HDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50548260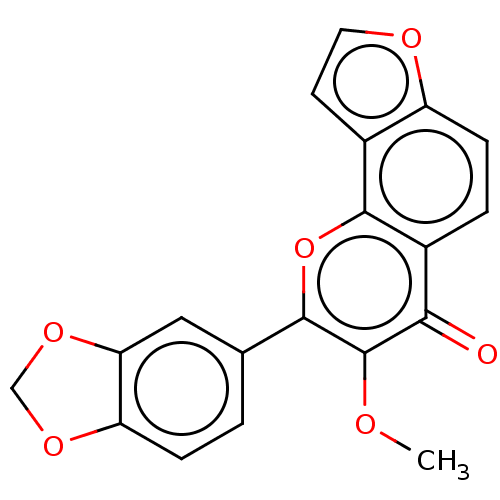 (Pongapin) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CYP1A1 expressed in HEK293 cells assessed as reduction in CYP1A1 mediated B[alpha]P-7,8- dihydrodiol induced-toxicity by measurin... | Citation and Details Article DOI: 10.1016/j.bmc.2018.11.013 BindingDB Entry DOI: 10.7270/Q20V8HDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||