 Found 1201 hits of ic50 for UniProtKB: P30556
Found 1201 hits of ic50 for UniProtKB: P30556 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049199
(4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C37H34N4O3S/c1-4-33-39-35-25(2)23-26(3)38-36(35)41(33)24-27-19-21-28(22-20-27)31-17-11-12-18-32(31)45(43,44)40-37(42)34(29-13-7-5-8-14-29)30-15-9-6-10-16-30/h5-23,34H,4,24H2,1-3H3,(H,40,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin II receptor, type 1 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50229504
(Saralasin)Show SMILES [H][C@]1(CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)CNC)C(C)C)C(C)C)C(=O)N[C@@H](C)C(O)=O |r| Show InChI InChI=1S/C42H65N13O10/c1-22(2)33(53-35(58)28(50-32(57)20-45-6)9-7-15-47-42(43)44)38(61)51-29(17-25-11-13-27(56)14-12-25)36(59)54-34(23(3)4)39(62)52-30(18-26-19-46-21-48-26)40(63)55-16-8-10-31(55)37(60)49-24(5)41(64)65/h11-14,19,21-24,28-31,33-34,45,56H,7-10,15-18,20H2,1-6H3,(H,46,48)(H,49,60)(H,50,57)(H,51,61)(H,52,62)(H,53,58)(H,54,59)(H,64,65)(H4,43,44,47)/t24-,28-,29-,30-,31-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity as antagonist of AII on guinea pig ileum |
J Med Chem 34: 2402-10 (1991)
BindingDB Entry DOI: 10.7270/Q2VD71PJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030692
(CHEMBL339672 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...)Show SMILES CCCCc1nn(-c2cc(NC(=O)CC)ccc2Cl)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1Cl Show InChI InChI=1S/C35H32Cl2FN5O5S/c1-3-5-14-32-40-43(30-20-24(17-18-28(30)37)39-33(44)4-2)35(46)42(32)21-23-16-15-22(19-29(23)38)25-10-7-9-13-31(25)49(47,48)41-34(45)26-11-6-8-12-27(26)36/h6-13,15-20H,3-5,14,21H2,1-2H3,(H,39,44)(H,41,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283231
(Biphenylsulfonylcarbamate compound | CHEMBL70935)Show SMILES CCCc1nc(CC)c(C(=O)OCC(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C36H40FN3O7S/c1-5-12-33-38-30(6-2)34(35(42)47-23-31(41)25-13-8-7-9-14-25)40(33)22-27-18-17-26(21-29(27)37)28-15-10-11-16-32(28)48(44,45)39-36(43)46-20-19-24(3)4/h7-11,13-18,21,24H,5-6,12,19-20,22-23H2,1-4H3,(H,39,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283235

(Biphenylsulfonylcarbamate compound | CHEMBL305017)Show SMILES CCCc1nc(CC)c(C(=O)OCCN(C(=O)c2ccccc2)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C43H47FN4O7S/c1-5-15-39-45-37(6-2)40(42(50)54-27-25-47(34-18-11-8-12-19-34)41(49)31-16-9-7-10-17-31)48(39)29-33-23-22-32(28-36(33)44)35-20-13-14-21-38(35)56(52,53)46-43(51)55-26-24-30(3)4/h7-14,16-23,28,30H,5-6,15,24-27,29H2,1-4H3,(H,46,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283237
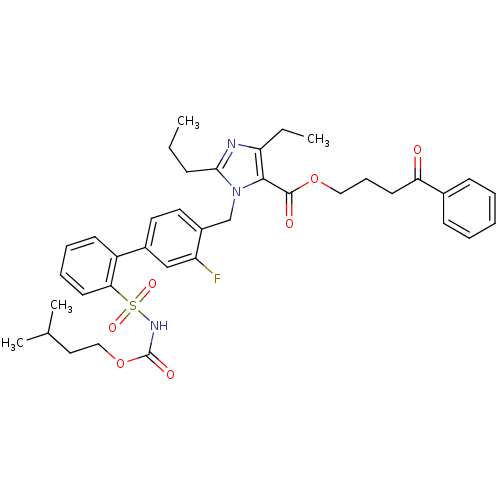
(Biphenylsulfonylcarbamate compound | CHEMBL70161)Show SMILES CCCc1nc(CC)c(C(=O)OCCCC(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C38H44FN3O7S/c1-5-13-35-40-32(6-2)36(37(44)48-22-12-17-33(43)27-14-8-7-9-15-27)42(35)25-29-20-19-28(24-31(29)39)30-16-10-11-18-34(30)50(46,47)41-38(45)49-23-21-26(3)4/h7-11,14-16,18-20,24,26H,5-6,12-13,17,21-23,25H2,1-4H3,(H,41,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283213

(Biphenylsulfonylcarbamate compound | CHEMBL307318)Show SMILES CCCCCOC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCC)nc(CC)c2C(=O)OCCC(=O)Nc2ccccc2)c(F)c1 Show InChI InChI=1S/C37H43FN4O7S/c1-4-7-13-22-49-37(45)41-50(46,47)32-18-12-11-17-29(32)26-19-20-27(30(38)24-26)25-42-33(14-5-2)40-31(6-3)35(42)36(44)48-23-21-34(43)39-28-15-9-8-10-16-28/h8-12,15-20,24H,4-7,13-14,21-23,25H2,1-3H3,(H,39,43)(H,41,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283223
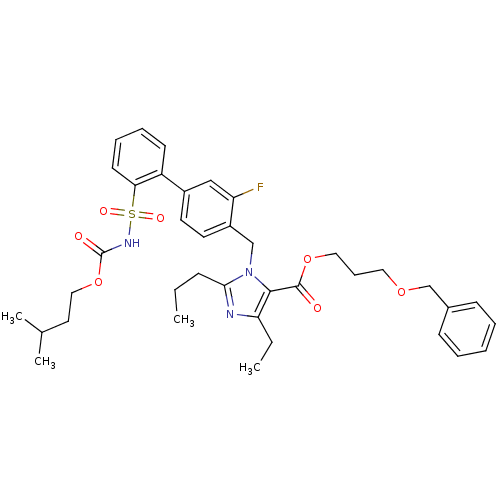
(Biphenylsulfonylcarbamate compound | CHEMBL70370)Show SMILES CCCc1nc(CC)c(C(=O)OCCCOCc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C38H46FN3O7S/c1-5-13-35-40-33(6-2)36(37(43)48-22-12-21-47-26-28-14-8-7-9-15-28)42(35)25-30-19-18-29(24-32(30)39)31-16-10-11-17-34(31)50(45,46)41-38(44)49-23-20-27(3)4/h7-11,14-19,24,27H,5-6,12-13,20-23,25-26H2,1-4H3,(H,41,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283219

(Biphenylsulfonylcarbamate compound | CHEMBL70843)Show SMILES CCCc1nc(CC)c(C(=O)OCCCN2C(=O)c3ccccc3C2=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C39H43FN4O8S/c1-5-12-34-41-32(6-2)35(38(47)51-21-11-20-43-36(45)29-14-7-8-15-30(29)37(43)46)44(34)24-27-18-17-26(23-31(27)40)28-13-9-10-16-33(28)53(49,50)42-39(48)52-22-19-25(3)4/h7-10,13-18,23,25H,5-6,11-12,19-22,24H2,1-4H3,(H,42,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50041969
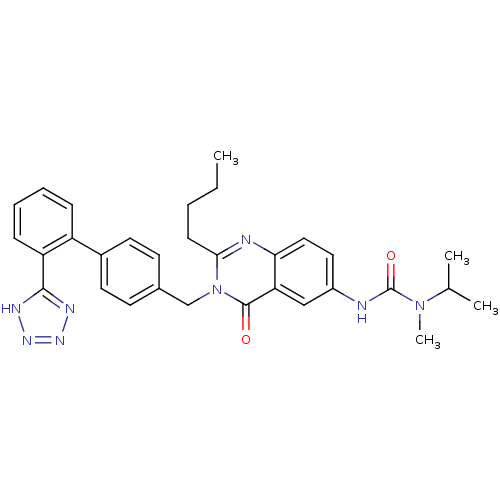
(3-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc2ccc(NC(=O)N(C)C(C)C)cc2c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H34N8O2/c1-5-6-11-28-33-27-17-16-23(32-31(41)38(4)20(2)3)18-26(27)30(40)39(28)19-21-12-14-22(15-13-21)24-9-7-8-10-25(24)29-34-36-37-35-29/h7-10,12-18,20H,5-6,11,19H2,1-4H3,(H,32,41)(H,34,35,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against angiotensin II receptor, type 1 |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50493576

(CHEMBL2435828)Show SMILES CCCc1nc2c(C)cc(NC(=O)CCc3ccccc3)cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C34H33N3O3/c1-3-9-31-36-33-23(2)20-27(35-32(38)19-16-24-10-5-4-6-11-24)21-30(33)37(31)22-25-14-17-26(18-15-25)28-12-7-8-13-29(28)34(39)40/h4-8,10-15,17-18,20-21H,3,9,16,19,22H2,1-2H3,(H,35,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis |
Eur J Med Chem 69: 44-54 (2013)
Article DOI: 10.1016/j.ejmech.2013.08.014
BindingDB Entry DOI: 10.7270/Q2PK0K32 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030684
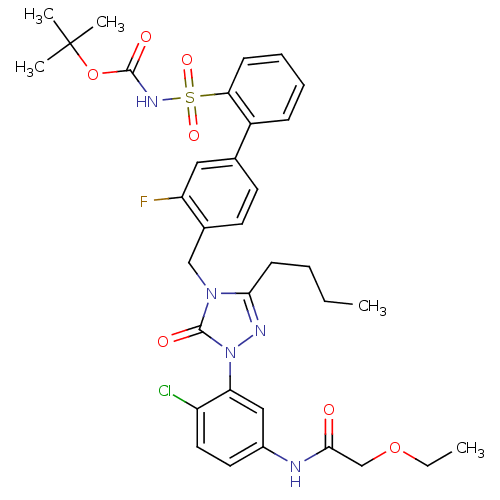
(CHEMBL339722 | N-{3-[3-Butyl-4-(3-fluoro-2-(N-t-bu...)Show SMILES CCCCc1nn(-c2cc(NC(=O)COCC)ccc2Cl)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OC(C)(C)C Show InChI InChI=1S/C34H39ClFN5O7S/c1-6-8-13-30-38-41(28-19-24(16-17-26(28)35)37-31(42)21-47-7-2)33(44)40(30)20-23-15-14-22(18-27(23)36)25-11-9-10-12-29(25)49(45,46)39-32(43)48-34(3,4)5/h9-12,14-19H,6-8,13,20-21H2,1-5H3,(H,37,42)(H,39,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283194

(Biphenylsulfonylcarbamate compound | CHEMBL69721)Show SMILES CCCc1nc(CC)c(C(=O)OCCCOc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C37H44FN3O7S/c1-5-13-34-39-32(6-2)35(36(42)47-22-12-21-46-29-14-8-7-9-15-29)41(34)25-28-19-18-27(24-31(28)38)30-16-10-11-17-33(30)49(44,45)40-37(43)48-23-20-26(3)4/h7-11,14-19,24,26H,5-6,12-13,20-23,25H2,1-4H3,(H,40,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283201
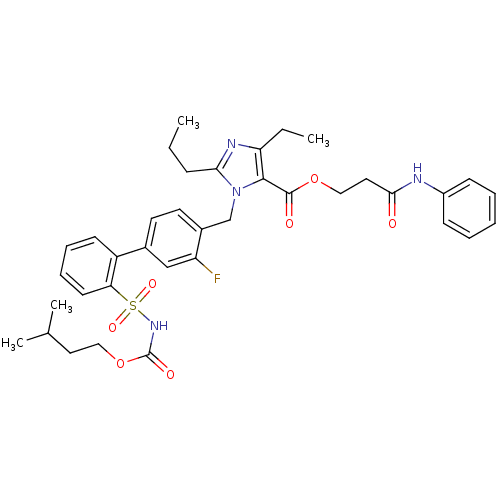
(Biphenylsulfonylcarbamate compound | CHEMBL305238)Show SMILES CCCc1nc(CC)c(C(=O)OCCC(=O)Nc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C37H43FN4O7S/c1-5-12-33-40-31(6-2)35(36(44)48-22-20-34(43)39-28-13-8-7-9-14-28)42(33)24-27-18-17-26(23-30(27)38)29-15-10-11-16-32(29)50(46,47)41-37(45)49-21-19-25(3)4/h7-11,13-18,23,25H,5-6,12,19-22,24H2,1-4H3,(H,39,43)(H,41,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283245
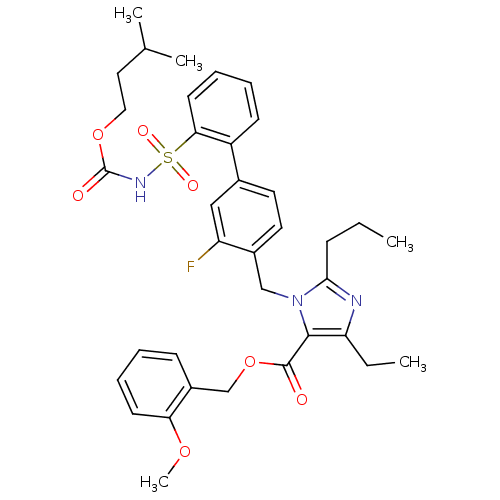
(Biphenylsulfonylcarbamate compound | CHEMBL70789)Show SMILES CCCc1nc(CC)c(C(=O)OCc2ccccc2OC)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C36H42FN3O7S/c1-6-12-33-38-30(7-2)34(35(41)47-23-27-13-8-10-15-31(27)45-5)40(33)22-26-18-17-25(21-29(26)37)28-14-9-11-16-32(28)48(43,44)39-36(42)46-20-19-24(3)4/h8-11,13-18,21,24H,6-7,12,19-20,22-23H2,1-5H3,(H,39,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030710
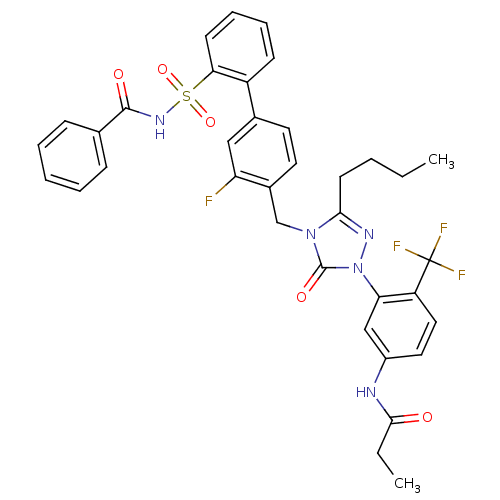
(CHEMBL332909 | N-{3-[4-(2'-Benzoylsulfamoyl-3-fluo...)Show SMILES CCCCc1nn(-c2cc(NC(=O)CC)ccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C36H33F4N5O5S/c1-3-5-15-32-42-45(30-21-26(41-33(46)4-2)18-19-28(30)36(38,39)40)35(48)44(32)22-25-17-16-24(20-29(25)37)27-13-9-10-14-31(27)51(49,50)43-34(47)23-11-7-6-8-12-23/h6-14,16-21H,3-5,15,22H2,1-2H3,(H,41,46)(H,43,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030698

(CHEMBL435811 | N-{3-[3-Butyl-4-(3-fluoro-2'-(N-t-b...)Show SMILES CCCCc1nn(-c2cc(NC(=O)CCOC)ccc2Cl)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OC(C)(C)C Show InChI InChI=1S/C34H39ClFN5O7S/c1-6-7-12-30-38-41(28-20-24(15-16-26(28)35)37-31(42)17-18-47-5)33(44)40(30)21-23-14-13-22(19-27(23)36)25-10-8-9-11-29(25)49(45,46)39-32(43)48-34(2,3)4/h8-11,13-16,19-20H,6-7,12,17-18,21H2,1-5H3,(H,37,42)(H,39,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030697

(CHEMBL339004 | N-Butyl-3-{3-butyl-4-[2'-(2-chloro-...)Show SMILES CCCCNC(=O)c1ccc(Cl)c(c1)-n1nc(CCCC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)c2ccccc2Cl)c1=O Show InChI InChI=1S/C37H36Cl2FN5O5S/c1-3-5-15-34-42-45(32-22-25(18-19-30(32)39)35(46)41-20-6-4-2)37(48)44(34)23-26-17-16-24(21-31(26)40)27-11-8-10-14-33(27)51(49,50)43-36(47)28-12-7-9-13-29(28)38/h7-14,16-19,21-22H,3-6,15,20,23H2,1-2H3,(H,41,46)(H,43,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030695

(CHEMBL340632 | N-Butyl-3-[3-butyl-4-(3-fluoro-2'-(...)Show SMILES CCCCNC(=O)c1ccc(Cl)c(c1)-n1nc(CCCC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)OC(C)(C)C)c1=O Show InChI InChI=1S/C35H41ClFN5O6S/c1-6-8-14-31-39-42(29-21-24(17-18-27(29)36)32(43)38-19-9-7-2)34(45)41(31)22-25-16-15-23(20-28(25)37)26-12-10-11-13-30(26)49(46,47)40-33(44)48-35(3,4)5/h10-13,15-18,20-21H,6-9,14,19,22H2,1-5H3,(H,38,43)(H,40,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50067536
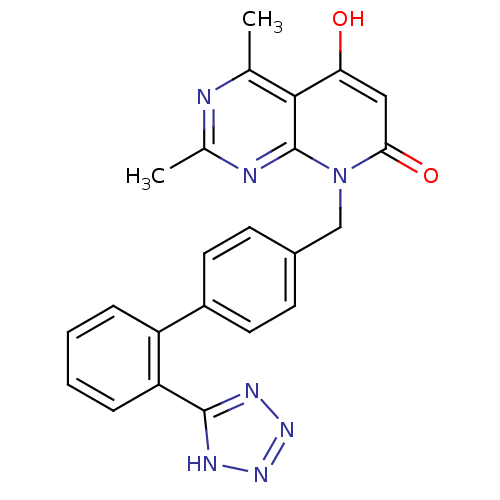
(5-Hydroxy-2,4-dimethyl-8-[2'-(1H-tetrazol-5-yl)-bi...)Show SMILES Cc1nc(C)c2c(O)cc(=O)n(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c2n1 Show InChI InChI=1S/C23H19N7O2/c1-13-21-19(31)11-20(32)30(23(21)25-14(2)24-13)12-15-7-9-16(10-8-15)17-5-3-4-6-18(17)22-26-28-29-27-22/h3-11,31H,12H2,1-2H3,(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition against Angiotensin II receptor, type 1 |
J Med Chem 46: 2261-70 (2003)
Article DOI: 10.1021/jm0204237
BindingDB Entry DOI: 10.7270/Q2154HSP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030689

(CHEMBL125008 | N-{4-Bromo-3-[3-butyl-4-(3-fluoro-2...)Show SMILES CCCCc1nn(-c2cc(NC(=O)c3ccccc3)ccc2Br)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OC(C)(C)C Show InChI InChI=1S/C37H37BrFN5O6S/c1-5-6-16-33-41-44(31-22-27(19-20-29(31)38)40-34(45)24-12-8-7-9-13-24)36(47)43(33)23-26-18-17-25(21-30(26)39)28-14-10-11-15-32(28)51(48,49)42-35(46)50-37(2,3)4/h7-15,17-22H,5-6,16,23H2,1-4H3,(H,40,45)(H,42,46) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283228
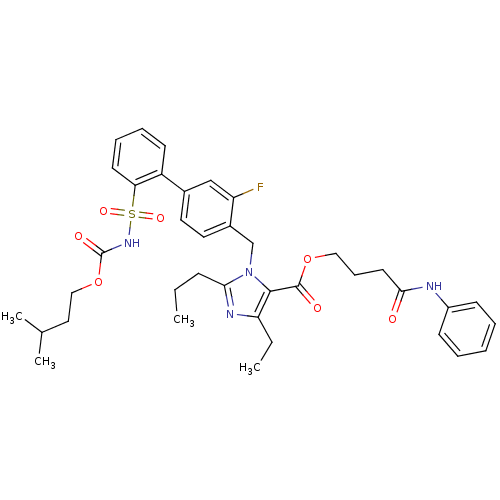
(Biphenylsulfonylcarbamate compound | CHEMBL430792)Show SMILES CCCc1nc(CC)c(C(=O)OCCCC(=O)Nc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C38H45FN4O7S/c1-5-13-34-41-32(6-2)36(37(45)49-22-12-18-35(44)40-29-14-8-7-9-15-29)43(34)25-28-20-19-27(24-31(28)39)30-16-10-11-17-33(30)51(47,48)42-38(46)50-23-21-26(3)4/h7-11,14-17,19-20,24,26H,5-6,12-13,18,21-23,25H2,1-4H3,(H,40,44)(H,42,46) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283225

(Biphenylsulfonylcarbamate compound | CHEMBL302964)Show SMILES CCCc1nc(CC)c(C(=O)OCCCCOc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C38H46FN3O7S/c1-5-14-35-40-33(6-2)36(37(43)48-23-13-12-22-47-30-15-8-7-9-16-30)42(35)26-29-20-19-28(25-32(29)39)31-17-10-11-18-34(31)50(45,46)41-38(44)49-24-21-27(3)4/h7-11,15-20,25,27H,5-6,12-14,21-24,26H2,1-4H3,(H,41,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283214

(Biphenylsulfonylcarbamate compound | CHEMBL71639)Show SMILES CCCc1nc(CC)c(C(=O)OCCCSc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C37H44FN3O6S2/c1-5-13-34-39-32(6-2)35(36(42)46-21-12-23-48-29-14-8-7-9-15-29)41(34)25-28-19-18-27(24-31(28)38)30-16-10-11-17-33(30)49(44,45)40-37(43)47-22-20-26(3)4/h7-11,14-19,24,26H,5-6,12-13,20-23,25H2,1-4H3,(H,40,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283204

(Biphenylsulfonylcarbamate compound | CHEMBL311625)Show SMILES CCCc1nc(CC)c(C(=O)OCCCS(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C37H44FN3O7S2/c1-5-13-34-39-32(6-2)35(36(42)47-21-12-23-49(44)29-14-8-7-9-15-29)41(34)25-28-19-18-27(24-31(28)38)30-16-10-11-17-33(30)50(45,46)40-37(43)48-22-20-26(3)4/h7-11,14-19,24,26H,5-6,12-13,20-23,25H2,1-4H3,(H,40,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283207
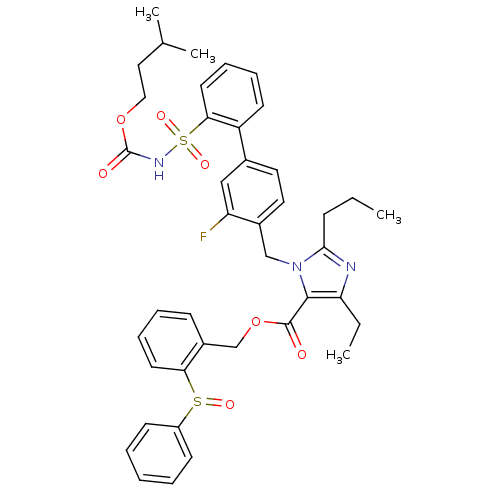
(Biphenylsulfonylcarbamate compound | CHEMBL73283)Show SMILES CCCc1nc(CC)c(C(=O)OCc2ccccc2S(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C41H44FN3O7S2/c1-5-14-38-43-35(6-2)39(40(46)52-27-31-15-10-12-19-36(31)53(48)32-16-8-7-9-17-32)45(38)26-30-22-21-29(25-34(30)42)33-18-11-13-20-37(33)54(49,50)44-41(47)51-24-23-28(3)4/h7-13,15-22,25,28H,5-6,14,23-24,26-27H2,1-4H3,(H,44,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283203

(Biphenylsulfonylcarbamate compound | CHEMBL70868)Show SMILES CCCCN(Cc1ccccc1)C(=O)CCCOC(=O)c1c(CC)nc(CCC)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C43H55FN4O7S/c1-6-9-25-47(29-32-17-11-10-12-18-32)40(49)21-15-26-54-42(50)41-37(8-3)45-39(16-7-2)48(41)30-34-23-22-33(28-36(34)44)35-19-13-14-20-38(35)56(52,53)46-43(51)55-27-24-31(4)5/h10-14,17-20,22-23,28,31H,6-9,15-16,21,24-27,29-30H2,1-5H3,(H,46,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283249

(Biphenylsulfonylcarbamate compound | CHEMBL308323)Show SMILES CCCc1nc(CC)c(C(=O)OCCCS(=O)(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C37H44FN3O8S2/c1-5-13-34-39-32(6-2)35(36(42)48-21-12-23-50(44,45)29-14-8-7-9-15-29)41(34)25-28-19-18-27(24-31(28)38)30-16-10-11-17-33(30)51(46,47)40-37(43)49-22-20-26(3)4/h7-11,14-19,24,26H,5-6,12-13,20-23,25H2,1-4H3,(H,40,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030711

(CHEMBL338027 | L-163958 | Pentanoic acid {4-bromo-...)Show SMILES CCCCC(=O)Nc1ccc(Br)c(c1)-n1nc(CC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)OC(C)(C)C)c1=O Show InChI InChI=1S/C33H37BrFN5O6S/c1-6-8-13-30(41)36-23-16-17-25(34)27(19-23)40-32(43)39(29(7-2)37-40)20-22-15-14-21(18-26(22)35)24-11-9-10-12-28(24)47(44,45)38-31(42)46-33(3,4)5/h9-12,14-19H,6-8,13,20H2,1-5H3,(H,36,41)(H,38,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin II receptor, type 1 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50038189

(4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C30H28N4O3S/c1-4-27-32-28-20(2)18-21(3)31-29(28)34(27)19-22-14-16-23(17-15-22)25-12-8-9-13-26(25)38(36,37)33-30(35)24-10-6-5-7-11-24/h5-18H,4,19H2,1-3H3,(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 1 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50229575

(CHEMBL2369937)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H](Cc1ccccc1)C(C)=O |r,wU:44.47,28.30,48.58,8.15,59.62,2.2,69.72,wD:4.4,18.26,32.43,76.78,(6.67,-4.52,;6.33,-6.02,;4.86,-6.48,;3.73,-5.43,;4.52,-7.98,;5.65,-9.02,;7.12,-8.57,;7.46,-7.07,;8.26,-9.61,;7.92,-11.11,;6.45,-11.57,;5.21,-10.65,;3.95,-11.54,;4.41,-13.01,;5.95,-13.03,;9.73,-9.16,;10.86,-10.2,;10.52,-11.7,;12.33,-9.74,;12.67,-8.24,;11.54,-7.2,;11.72,-5.67,;10.32,-5.02,;9.27,-6.15,;10.03,-7.5,;13.46,-10.79,;14.93,-10.33,;15.27,-8.83,;16.06,-11.38,;17.53,-10.92,;18.66,-11.96,;18.32,-13.47,;20.13,-11.51,;20.47,-10,;19.34,-8.96,;17.87,-9.42,;16.74,-8.37,;17.08,-6.87,;15.95,-5.82,;18.55,-6.41,;19.68,-7.46,;21.26,-12.55,;22.73,-12.09,;23.07,-10.59,;23.87,-13.14,;25.34,-12.68,;26.47,-13.73,;26.13,-15.23,;27.94,-13.27,;29.07,-14.31,;28.73,-15.82,;27.26,-16.27,;26.92,-17.78,;25.45,-18.23,;24.32,-17.19,;25.11,-19.74,;28.28,-11.77,;29.75,-11.31,;30.88,-12.36,;30.09,-9.81,;31.56,-9.35,;31.9,-7.85,;30.77,-6.8,;33.37,-7.39,;28.96,-8.76,;27.49,-9.22,;23.53,-14.64,;22.06,-15.1,;24.66,-15.69,;15.72,-12.88,;16.85,-13.92,;14.25,-13.34,;13.12,-12.29,;3.05,-8.44,;2.71,-9.94,;1.92,-7.39,;.45,-7.85,;-.68,-6.8,;-2.15,-7.26,;-3.28,-6.22,;-4.75,-6.67,;-5.09,-8.18,;-3.96,-9.22,;-2.49,-8.76,;.11,-9.35,;-1.36,-9.81,;1.24,-10.4,)| Show InChI InChI=1S/C59H86N16O12/c1-9-33(5)49(57(86)69-42(35(7)76)23-36-15-12-11-13-16-36)75-55(84)46(26-39-29-64-31-67-39)70-53(82)45(25-38-28-63-30-66-38)72-58(87)50(34(6)10-2)74-54(83)44(24-37-18-20-40(77)21-19-37)71-56(85)48(32(3)4)73-51(80)41(17-14-22-65-59(60)61)68-52(81)43(62-8)27-47(78)79/h11-13,15-16,18-21,28-34,41-46,48-50,62,77H,9-10,14,17,22-27H2,1-8H3,(H,63,66)(H,64,67)(H,68,81)(H,69,86)(H,70,82)(H,71,85)(H,72,87)(H,73,80)(H,74,83)(H,75,84)(H,78,79)(H4,60,61,65)/t33-,34-,41-,42-,43-,44-,45-,46-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Exploratory Chemistry Merck Sharp and Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin receptor from rabbit aorta |
J Med Chem 34: 2919-22 (1991)
BindingDB Entry DOI: 10.7270/Q26Q20H6 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030688
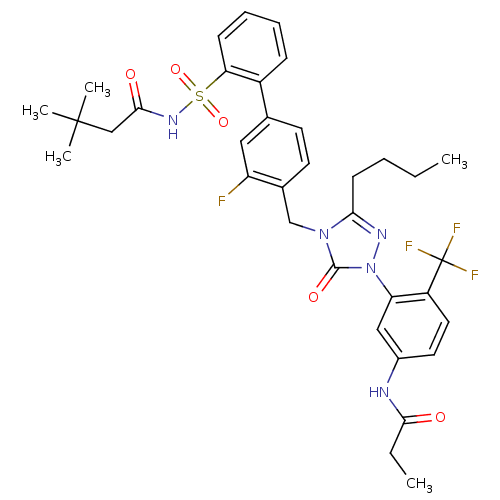
(CHEMBL122917 | N-(3-{3-Butyl-4-[2'-(3,3-dimethyl-b...)Show SMILES CCCCc1nn(-c2cc(NC(=O)CC)ccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)CC(C)(C)C Show InChI InChI=1S/C35H39F4N5O5S/c1-6-8-13-30-41-44(28-19-24(40-31(45)7-2)16-17-26(28)35(37,38)39)33(47)43(30)21-23-15-14-22(18-27(23)36)25-11-9-10-12-29(25)50(48,49)42-32(46)20-34(3,4)5/h9-12,14-19H,6-8,13,20-21H2,1-5H3,(H,40,45)(H,42,46) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030712

(2-Ethoxy-N-{3-[4-(3-fluoro-2'-(N-t-butyloxycarbony...)Show SMILES CCCc1nn(-c2cc(NC(=O)COCC)ccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OC(C)(C)C Show InChI InChI=1S/C34H37F4N5O7S/c1-6-10-29-40-43(27-18-23(39-30(44)20-49-7-2)15-16-25(27)34(36,37)38)32(46)42(29)19-22-14-13-21(17-26(22)35)24-11-8-9-12-28(24)51(47,48)41-31(45)50-33(3,4)5/h8-9,11-18H,6-7,10,19-20H2,1-5H3,(H,39,44)(H,41,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283200
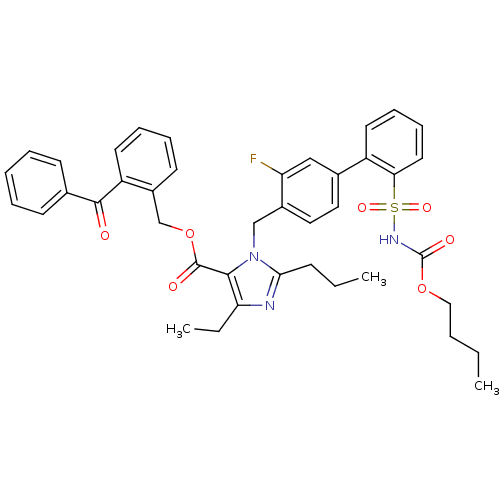
(Biphenylsulfonylcarbamate compound | CHEMBL71172)Show SMILES CCCCOC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCC)nc(CC)c2C(=O)OCc2ccccc2C(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C41H42FN3O7S/c1-4-7-24-51-41(48)44-53(49,50)36-21-14-13-19-32(36)29-22-23-30(34(42)25-29)26-45-37(15-5-2)43-35(6-3)38(45)40(47)52-27-31-18-11-12-20-33(31)39(46)28-16-9-8-10-17-28/h8-14,16-23,25H,4-7,15,24,26-27H2,1-3H3,(H,44,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283209
(Biphenylsulfonylcarbamate compound | CHEMBL303427)Show SMILES CCCc1nc(CC)c(C(=O)OCCN(C(=O)OCCC(C)C)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C42H53FN4O8S/c1-7-14-38-44-36(8-2)39(40(48)53-26-23-46(33-15-10-9-11-16-33)42(50)55-25-22-30(5)6)47(38)28-32-20-19-31(27-35(32)43)34-17-12-13-18-37(34)56(51,52)45-41(49)54-24-21-29(3)4/h9-13,15-20,27,29-30H,7-8,14,21-26,28H2,1-6H3,(H,45,49) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283215
(Biphenylsulfonylcarbamate compound | CHEMBL311253)Show SMILES CCCc1nc(CC)c(C(=O)OCCN2C(=O)c3ccccc3C2=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C38H41FN4O8S/c1-5-11-33-40-31(6-2)34(37(46)50-21-19-42-35(44)28-13-7-8-14-29(28)36(42)45)43(33)23-26-17-16-25(22-30(26)39)27-12-9-10-15-32(27)52(48,49)41-38(47)51-20-18-24(3)4/h7-10,12-17,22,24H,5-6,11,18-21,23H2,1-4H3,(H,41,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030713
(5-n-Butyl-4-[[2'-[N-(tert-butoxycarbonyl)sulfamoyl...)Show SMILES CCCCc1nn(-c2cc(NC(=O)CC)ccc2Cl)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)OC(C)(C)C Show InChI InChI=1S/C33H38ClN5O6S/c1-6-8-13-29-36-39(27-20-24(18-19-26(27)34)35-30(40)7-2)32(42)38(29)21-22-14-16-23(17-15-22)25-11-9-10-12-28(25)46(43,44)37-31(41)45-33(3,4)5/h9-12,14-20H,6-8,13,21H2,1-5H3,(H,35,40)(H,37,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030815
(CHEMBL404594)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)[C@@H](C)CC)C(O)=O |wU:8.7,25.27,wD:41.44,4.4,15.16,29.31,45.48,2.2,62.65,(8.44,-18.06,;9.36,-17.24,;9.05,-15.73,;7.88,-15.34,;10.19,-14.7,;9.88,-13.19,;11.03,-12.16,;12.2,-12.55,;10.71,-10.66,;11.74,-9.51,;10.97,-8.18,;9.46,-8.5,;9.33,-10.02,;7.99,-10.79,;7.99,-12.02,;6.66,-10.02,;5.32,-10.79,;5.32,-12.33,;4.07,-13.22,;4.55,-14.68,;6.09,-14.68,;6.56,-13.21,;6.66,-8.48,;5.33,-7.71,;4.43,-8.22,;5.33,-6.17,;3.99,-5.39,;4,-3.85,;5.06,-3.24,;2.66,-3.08,;1.33,-3.85,;-.01,-3.08,;-1.34,-3.84,;-2.67,-3.07,;-2.67,-1.53,;-3.73,-.91,;-1.33,-.76,;,-1.54,;2.67,-1.54,;4,-.77,;5.07,-1.39,;4.01,.77,;5.35,1.53,;5.36,3.07,;4.29,3.69,;6.69,3.84,;6.7,5.37,;8.04,6.14,;8.04,7.68,;9.38,8.44,;9.39,9.99,;10.45,10.6,;8.32,10.61,;8.03,3.06,;8.02,1.52,;6.94,.91,;9.35,.74,;9.34,-.8,;10.22,-1.32,;2.68,1.54,;1.61,.93,;2.69,2.78,;6.66,-5.4,;7.73,-6.01,;6.66,-3.86,;7.73,-3.24,;11.66,-15.17,;11.91,-16.38,;12.58,-14.35,)| Show InChI InChI=1S/C46H73N13O10/c1-8-26(5)37(43(66)55-33(21-29-22-50-24-52-29)44(67)59-19-11-13-34(59)41(64)58-38(45(68)69)27(6)9-2)57-40(63)32(20-28-14-16-30(60)17-15-28)54-42(65)36(25(3)4)56-39(62)31(53-35(61)23-49-7)12-10-18-51-46(47)48/h14-17,22,24-27,31-34,36-38,49,60H,8-13,18-21,23H2,1-7H3,(H,50,52)(H,53,61)(H,54,65)(H,55,66)(H,56,62)(H,57,63)(H,58,64)(H,68,69)(H4,47,48,51)/t26-,27-,31-,32-,33-,34-,36-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity as antagonist of AII on guinea pig ileum |
J Med Chem 34: 2402-10 (1991)
BindingDB Entry DOI: 10.7270/Q2VD71PJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030707
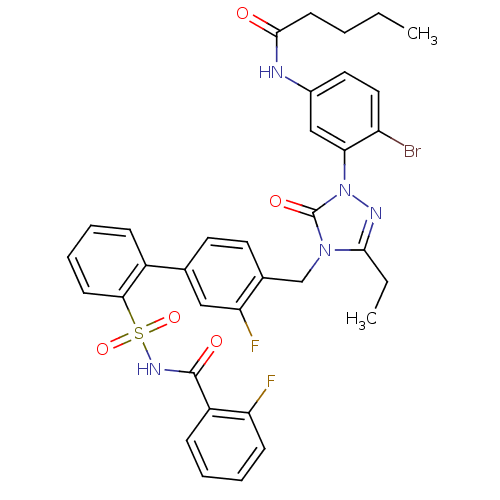
(CHEMBL122273 | Pentanoic acid (4-bromo-3-{3-ethyl-...)Show SMILES CCCCC(=O)Nc1ccc(Br)c(c1)-n1nc(CC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)c2ccccc2F)c1=O Show InChI InChI=1S/C35H32BrF2N5O5S/c1-3-5-14-33(44)39-24-17-18-27(36)30(20-24)43-35(46)42(32(4-2)40-43)21-23-16-15-22(19-29(23)38)25-10-7-9-13-31(25)49(47,48)41-34(45)26-11-6-8-12-28(26)37/h6-13,15-20H,3-5,14,21H2,1-2H3,(H,39,44)(H,41,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030691

(CHEMBL122380 | Pentanoic acid (4-bromo-3-{4-[2'-(2...)Show SMILES CCCCC(=O)Nc1ccc(Br)c(c1)-n1nc(CC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)c2cc(F)ccc2F)c1=O Show InChI InChI=1S/C35H31BrF3N5O5S/c1-3-5-10-33(45)40-24-14-15-27(36)30(19-24)44-35(47)43(32(4-2)41-44)20-22-12-11-21(17-29(22)39)25-8-6-7-9-31(25)50(48,49)42-34(46)26-18-23(37)13-16-28(26)38/h6-9,11-19H,3-5,10,20H2,1-2H3,(H,40,45)(H,42,46) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030687
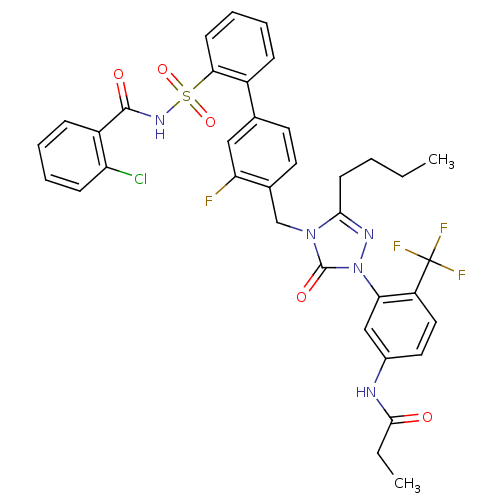
(CHEMBL339256 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...)Show SMILES CCCCc1nn(-c2cc(NC(=O)CC)ccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1Cl Show InChI InChI=1S/C36H32ClF4N5O5S/c1-3-5-14-32-43-46(30-20-24(42-33(47)4-2)17-18-27(30)36(39,40)41)35(49)45(32)21-23-16-15-22(19-29(23)38)25-10-7-9-13-31(25)52(50,51)44-34(48)26-11-6-8-12-28(26)37/h6-13,15-20H,3-5,14,21H2,1-2H3,(H,42,47)(H,44,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in human adrenal membrane preparations. For this assay, only 0.02%BSA was presen... |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030722

(3-[3-Butyl-4-(3-fluoro-2'-(N-t-butyloxycarbonyl)-S...)Show SMILES CCCCc1nn(-c2cc(ccc2Cl)C(=O)NCCOC)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OC(C)(C)C Show InChI InChI=1S/C34H39ClFN5O7S/c1-6-7-12-30-38-41(28-20-23(15-16-26(28)35)31(42)37-17-18-47-5)33(44)40(30)21-24-14-13-22(19-27(24)36)25-10-8-9-11-29(25)49(45,46)39-32(43)48-34(2,3)4/h8-11,13-16,19-20H,6-7,12,17-18,21H2,1-5H3,(H,37,42)(H,39,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030702
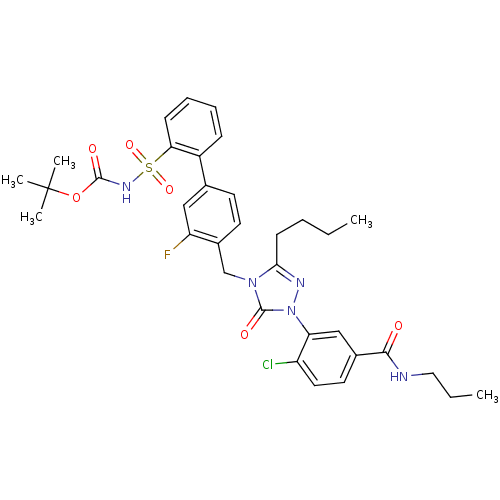
(3-[3-Butyl-4-(3-fluoro-2'-(N-t-butyloxycarbonyl)-S...)Show SMILES CCCCc1nn(-c2cc(ccc2Cl)C(=O)NCCC)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OC(C)(C)C Show InChI InChI=1S/C34H39ClFN5O6S/c1-6-8-13-30-38-41(28-20-23(16-17-26(28)35)31(42)37-18-7-2)33(44)40(30)21-24-15-14-22(19-27(24)36)25-11-9-10-12-29(25)48(45,46)39-32(43)47-34(3,4)5/h9-12,14-17,19-20H,6-8,13,18,21H2,1-5H3,(H,37,42)(H,39,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030690

(CHEMBL339113 | N-{3-[3-Butyl-4-(3-fluoro-2'-(N-t-b...)Show SMILES CCCCc1nn(-c2cc(NC(=O)CC)ccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OC(C)(C)C Show InChI InChI=1S/C34H37F4N5O6S/c1-6-8-13-29-40-43(27-19-23(39-30(44)7-2)16-17-25(27)34(36,37)38)32(46)42(29)20-22-15-14-21(18-26(22)35)24-11-9-10-12-28(24)50(47,48)41-31(45)49-33(3,4)5/h9-12,14-19H,6-8,13,20H2,1-5H3,(H,39,44)(H,41,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283248
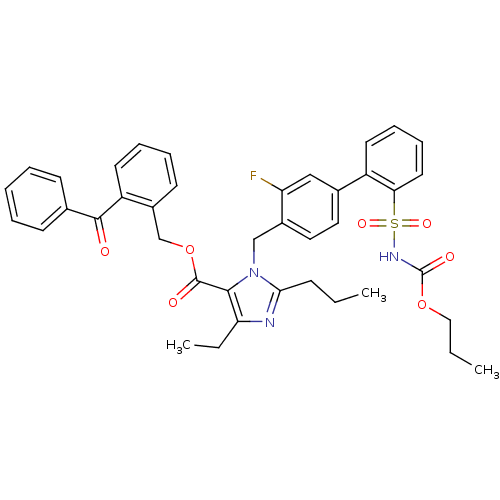
(Biphenylsulfonylcarbamate compound | CHEMBL70726)Show SMILES CCCOC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCC)nc(CC)c2C(=O)OCc2ccccc2C(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C40H40FN3O7S/c1-4-14-36-42-34(6-3)37(39(46)51-26-30-17-10-11-19-32(30)38(45)27-15-8-7-9-16-27)44(36)25-29-22-21-28(24-33(29)41)31-18-12-13-20-35(31)52(48,49)43-40(47)50-23-5-2/h7-13,15-22,24H,4-6,14,23,25-26H2,1-3H3,(H,43,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283238
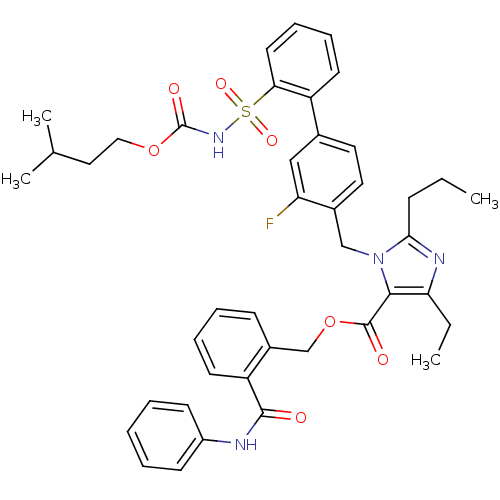
(Biphenylsulfonylcarbamate compound | CHEMBL70867)Show SMILES CCCc1nc(CC)c(C(=O)OCc2ccccc2C(=O)Nc2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C42H45FN4O7S/c1-5-14-38-45-36(6-2)39(41(49)54-27-31-15-10-11-19-34(31)40(48)44-32-16-8-7-9-17-32)47(38)26-30-22-21-29(25-35(30)43)33-18-12-13-20-37(33)55(51,52)46-42(50)53-24-23-28(3)4/h7-13,15-22,25,28H,5-6,14,23-24,26-27H2,1-4H3,(H,44,48)(H,46,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283198
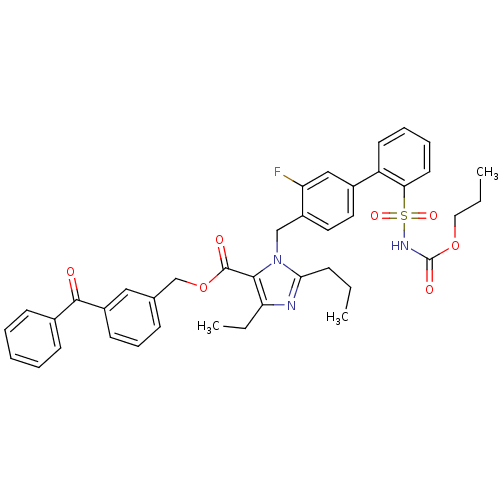
(Biphenylsulfonylcarbamate compound | CHEMBL311018)Show SMILES CCCOC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCC)nc(CC)c2C(=O)OCc2cccc(c2)C(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C40H40FN3O7S/c1-4-13-36-42-34(6-3)37(39(46)51-26-27-14-12-17-30(23-27)38(45)28-15-8-7-9-16-28)44(36)25-31-21-20-29(24-33(31)41)32-18-10-11-19-35(32)52(48,49)43-40(47)50-22-5-2/h7-12,14-21,23-24H,4-6,13,22,25-26H2,1-3H3,(H,43,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283220

(Biphenylsulfonylcarbamate compound | CHEMBL302579)Show SMILES CCCc1nc(CC)c(C(=O)OCc2ccccc2NC(=O)c2ccccc2)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)OCCC(C)C Show InChI InChI=1S/C42H45FN4O7S/c1-5-14-38-44-35(6-2)39(41(49)54-27-32-17-10-12-19-36(32)45-40(48)29-15-8-7-9-16-29)47(38)26-31-22-21-30(25-34(31)43)33-18-11-13-20-37(33)55(51,52)46-42(50)53-24-23-28(3)4/h7-13,15-22,25,28H,5-6,14,23-24,26-27H2,1-4H3,(H,45,48)(H,46,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2235-2240 (1994)
Article DOI: 10.1016/S0960-894X(00)80077-3
BindingDB Entry DOI: 10.7270/Q2DZ088W |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50031491

(2-propyl-4-(methythio)-1-[[[2'-[(propylamino)carbo...)Show SMILES CCCNC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCC)nc(SC)c2C(O)=O)cc1 Show InChI InChI=1S/C25H30N4O5S2/c1-4-8-21-27-23(35-3)22(24(30)31)29(21)16-17-11-13-18(14-12-17)19-9-6-7-10-20(19)36(33,34)28-25(32)26-15-5-2/h6-7,9-14H,4-5,8,15-16H2,1-3H3,(H,30,31)(H2,26,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin II receptor, type 1 |
J Med Chem 39: 625-56 (1996)
Article DOI: 10.1021/jm9504722
BindingDB Entry DOI: 10.7270/Q29P3299 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50009718
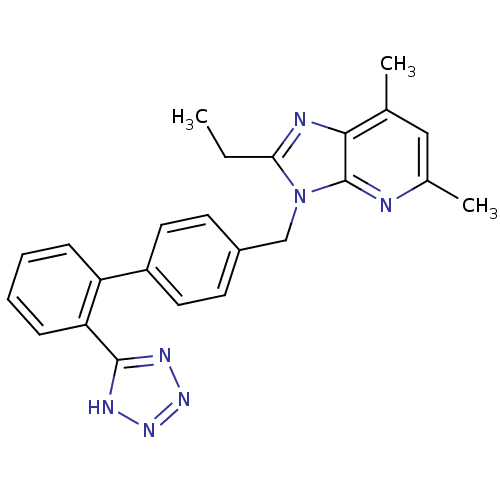
(2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...)Show SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23N7/c1-4-21-26-22-15(2)13-16(3)25-24(22)31(21)14-17-9-11-18(12-10-17)19-7-5-6-8-20(19)23-27-29-30-28-23/h5-13H,4,14H2,1-3H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 1 receptor |
J Med Chem 54: 2529-91 (2011)
Article DOI: 10.1021/jm1013693
BindingDB Entry DOI: 10.7270/Q24M95PH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data