

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM23954 (5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114154 BindingDB Entry DOI: 10.7270/Q2542SKF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50169552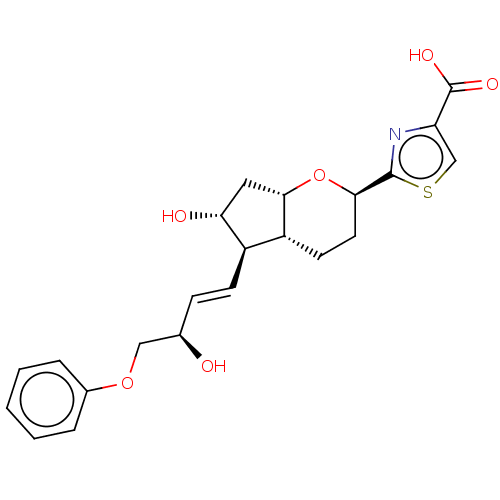 (CHEMBL3804978) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as increase in intracellular calcium level by fluorescence based analysis | ACS Med Chem Lett 7: 306-11 (2016) Article DOI: 10.1021/acsmedchemlett.5b00455 BindingDB Entry DOI: 10.7270/Q2125VKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as increase in intracellular calcium level by fluorescence based analysis | ACS Med Chem Lett 7: 306-11 (2016) Article DOI: 10.1021/acsmedchemlett.5b00455 BindingDB Entry DOI: 10.7270/Q2125VKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM62356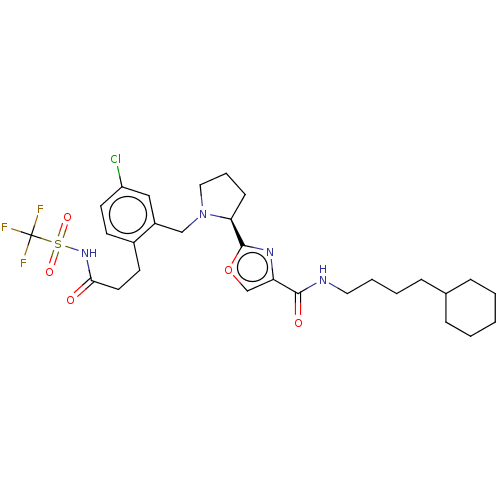 (US9422273, 12b) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 20 | n/a | n/a | 7.4 | 37 |
ALLERGAN, INC. US Patent | Assay Description Ca2+ signaling studies were performed using a FLIPR TETRA system (Molecular Devices, Sunnyvale, Calif., USA) in the 384-format. This is a high-throug... | US Patent US9422273 (2016) BindingDB Entry DOI: 10.7270/Q2DZ076K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM240747 (US9422273, 12e) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 20 | n/a | n/a | 7.4 | 37 |
ALLERGAN, INC. US Patent | Assay Description Ca2+ signaling studies were performed using a FLIPR TETRA system (Molecular Devices, Sunnyvale, Calif., USA) in the 384-format. This is a high-throug... | US Patent US9422273 (2016) BindingDB Entry DOI: 10.7270/Q2DZ076K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM184630 (US9156810, (52, 53)) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 27 | n/a | n/a | 7.4 | 4 |
Allergan, Inc. US Patent | Assay Description HEK-293 cells stably expressing the human or feline FP receptor, or EP1, EP2, EP3, or EP4 receptors were washed with TME buffer, scraped from the bot... | US Patent US9156810 (2015) BindingDB Entry DOI: 10.7270/Q2PV6J5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM240744 (US9422273, 12a) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 47 | n/a | n/a | 7.4 | 37 |
ALLERGAN, INC. US Patent | Assay Description Ca2+ signaling studies were performed using a FLIPR TETRA system (Molecular Devices, Sunnyvale, Calif., USA) in the 384-format. This is a high-throug... | US Patent US9422273 (2016) BindingDB Entry DOI: 10.7270/Q2DZ076K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM240746 (US9422273, 12d) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 63 | n/a | n/a | 7.4 | 37 |
ALLERGAN, INC. US Patent | Assay Description Ca2+ signaling studies were performed using a FLIPR TETRA system (Molecular Devices, Sunnyvale, Calif., USA) in the 384-format. This is a high-throug... | US Patent US9422273 (2016) BindingDB Entry DOI: 10.7270/Q2DZ076K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM240745 (US9422273, 12c) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 70 | n/a | n/a | 7.4 | 37 |
ALLERGAN, INC. US Patent | Assay Description Ca2+ signaling studies were performed using a FLIPR TETRA system (Molecular Devices, Sunnyvale, Calif., USA) in the 384-format. This is a high-throug... | US Patent US9422273 (2016) BindingDB Entry DOI: 10.7270/Q2DZ076K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50169550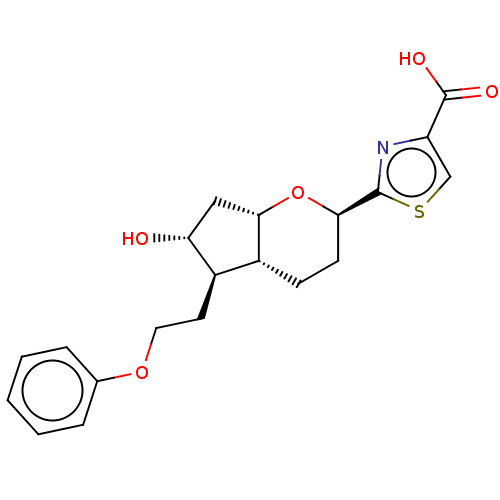 (CHEMBL3806130) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 97 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as increase in intracellular calcium level by fluorescence based analysis | ACS Med Chem Lett 7: 306-11 (2016) Article DOI: 10.1021/acsmedchemlett.5b00455 BindingDB Entry DOI: 10.7270/Q2125VKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50169548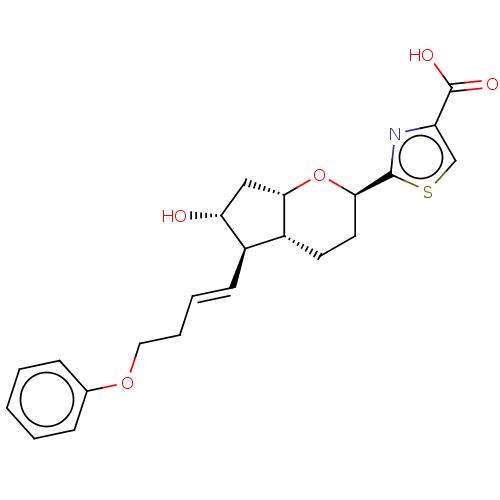 (CHEMBL3805176) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as increase in intracellular calcium level by fluorescence based analysis | ACS Med Chem Lett 7: 306-11 (2016) Article DOI: 10.1021/acsmedchemlett.5b00455 BindingDB Entry DOI: 10.7270/Q2125VKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50594971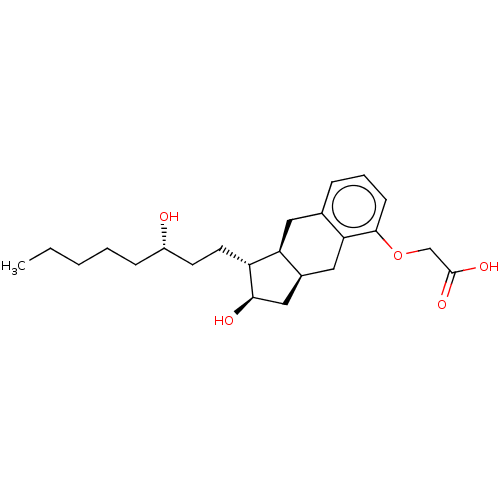 (15-AU-81 | 15AU81 | CHEBI:50861 | L-606 | LRX -15 ...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 285 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114154 BindingDB Entry DOI: 10.7270/Q2542SKF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50090136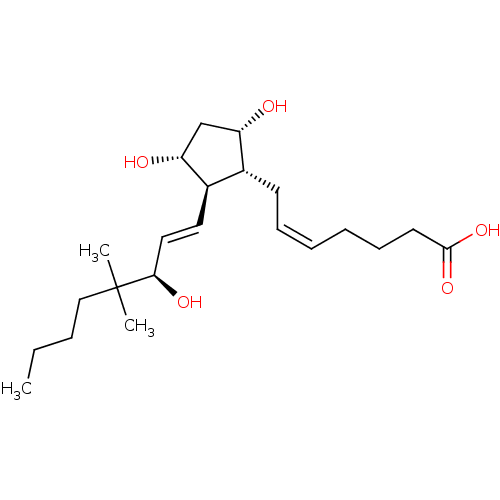 ((Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-((E)-(R)-3-hy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Functional activity in RAT-1cells, transiently-transfected with human Prostaglandin E receptor EP1 (% of control ligand, 17-phi-PGE2=80%) | Bioorg Med Chem Lett 10: 1519-22 (2000) BindingDB Entry DOI: 10.7270/Q28W3CJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50035622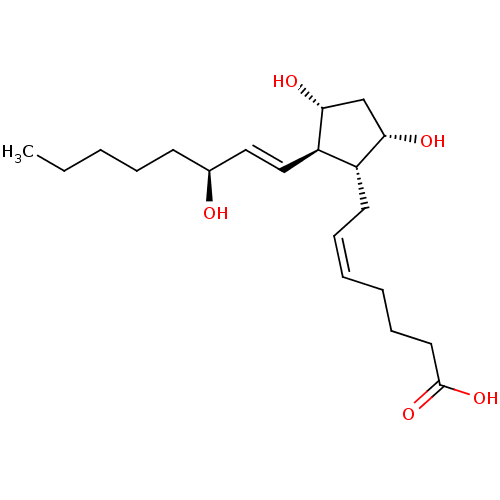 ((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Functional activity in RAT-1cells, transiently-transfected with human Prostaglandin E receptor EP1 (% of control ligand, 17-phi-PGE2=80%) | Bioorg Med Chem Lett 10: 1519-22 (2000) BindingDB Entry DOI: 10.7270/Q28W3CJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50169546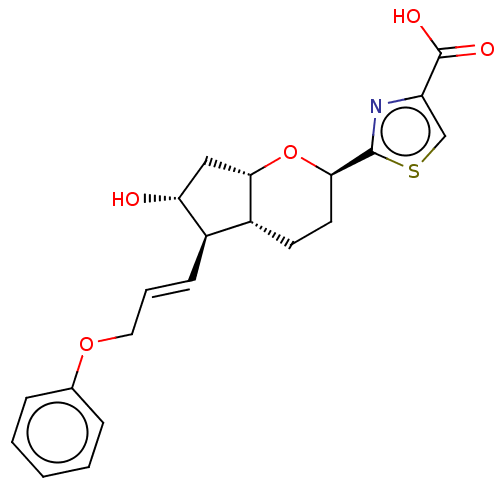 (CHEMBL3805693) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as increase in intracellular calcium level by fluorescence based analysis | ACS Med Chem Lett 7: 306-11 (2016) Article DOI: 10.1021/acsmedchemlett.5b00455 BindingDB Entry DOI: 10.7270/Q2125VKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50085910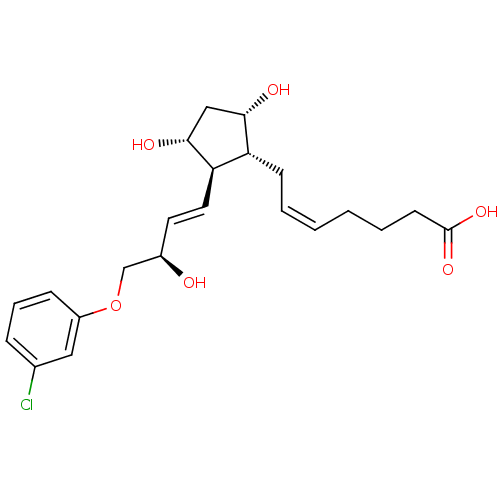 ((Z)-7-{(1R,2R,3R,5S)-2-[(E)-(R)-4-(3-Chloro-phenox...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 760 | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Functional activity in RAT-1cells, transiently-transfected with human Prostaglandin E receptor EP1 (% of control ligand, 17-phi-PGE2=80%) | Bioorg Med Chem Lett 10: 1519-22 (2000) BindingDB Entry DOI: 10.7270/Q28W3CJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50169549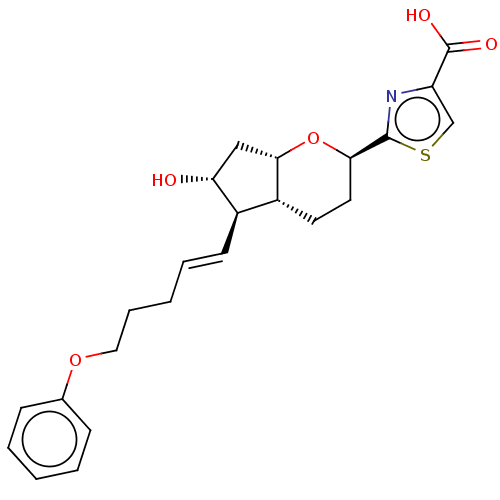 (CHEMBL3805169) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 970 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as increase in intracellular calcium level by fluorescence based analysis | ACS Med Chem Lett 7: 306-11 (2016) Article DOI: 10.1021/acsmedchemlett.5b00455 BindingDB Entry DOI: 10.7270/Q2125VKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM184630 (US9156810, (52, 53)) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 1.02E+3 | n/a | n/a | 7.4 | 4 |
Allergan, Inc. US Patent | Assay Description HEK-293 cells stably expressing the human or feline FP receptor, or EP1, EP2, EP3, or EP4 receptors were washed with TME buffer, scraped from the bot... | US Patent US9156810 (2015) BindingDB Entry DOI: 10.7270/Q2PV6J5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50454060 (CHEMBL4208379) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at recombinant human EP1 receptor expressed in CHO cells assessed as increase in intracellular calcium level measured at 3 secs time... | Bioorg Med Chem 26: 200-214 (2018) Article DOI: 10.1016/j.bmc.2017.11.035 BindingDB Entry DOI: 10.7270/Q2FF3VZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50545401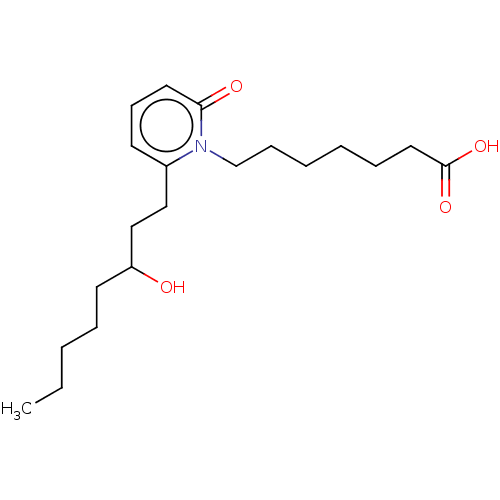 (CHEMBL4649582) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a |
Inception Sciences Canada Inc. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in HEK293 cells by calcium-5 dye based FLIPR assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127104 BindingDB Entry DOI: 10.7270/Q29C7214 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50169547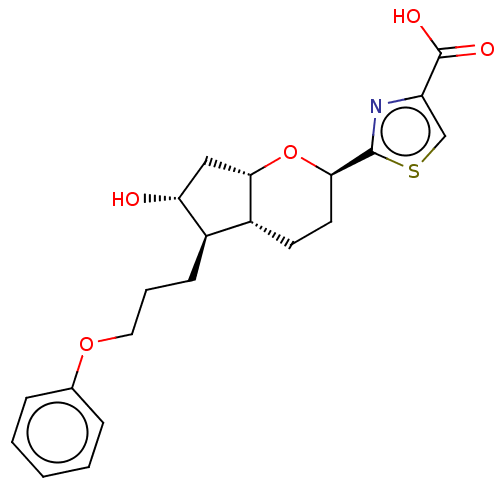 (CHEMBL3805044) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as increase in intracellular calcium level by fluorescence based analysis | ACS Med Chem Lett 7: 306-11 (2016) Article DOI: 10.1021/acsmedchemlett.5b00455 BindingDB Entry DOI: 10.7270/Q2125VKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50156473 (CHEMBL3793724) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as intracellular Ca2+ level by fluorescence analysis | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50156472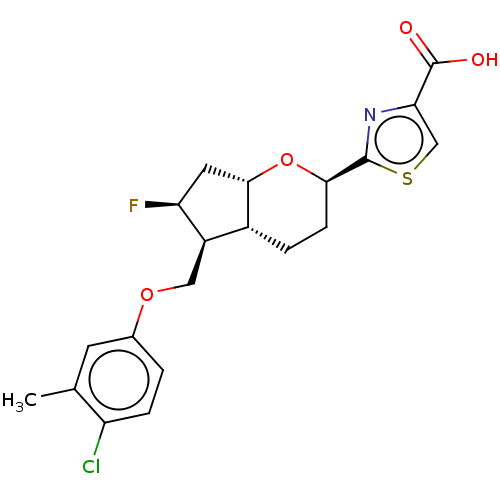 (CHEMBL3793167) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as intracellular Ca2+ level by fluorescence analysis | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50156544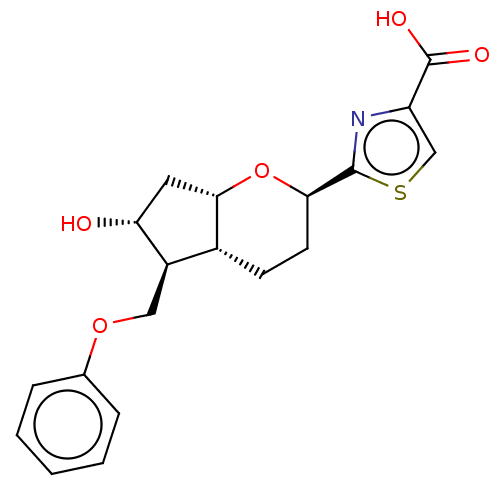 (CHEMBL3793209) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as increase in intracellular calcium level by fluorescence based analysis | ACS Med Chem Lett 7: 306-11 (2016) Article DOI: 10.1021/acsmedchemlett.5b00455 BindingDB Entry DOI: 10.7270/Q2125VKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50156530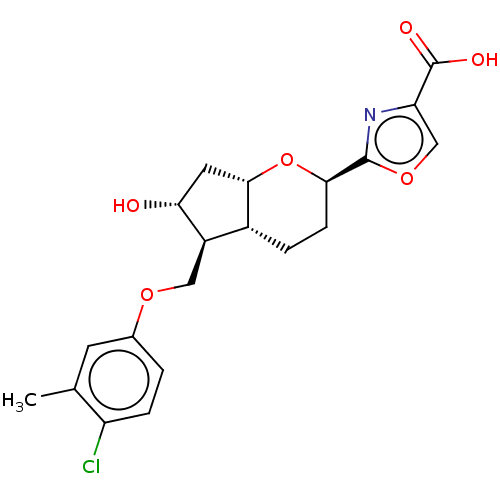 (CHEMBL3793009) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as intracellular Ca2+ level by fluorescence analysis | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50156539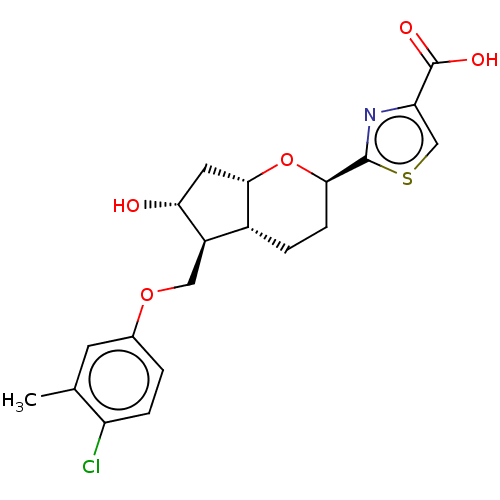 (CHEMBL3793892) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as intracellular Ca2+ level by fluorescence analysis | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50085914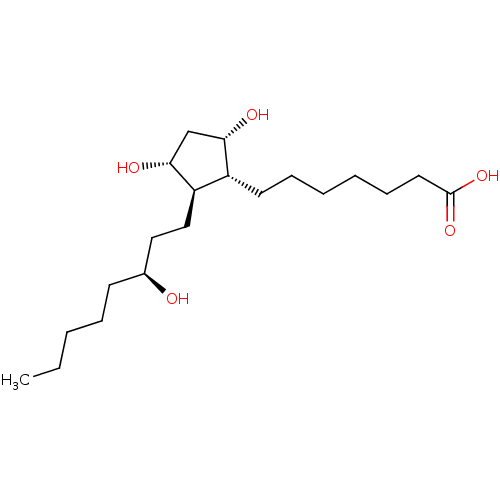 (7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-((S)-3-hydroxy-oc...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Functional activity in RAT-1cells, transiently-transfected with human Prostaglandin E receptor EP1 (% of control ligand, 17-phi-PGE2<50%) | Bioorg Med Chem Lett 10: 1519-22 (2000) BindingDB Entry DOI: 10.7270/Q28W3CJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50156474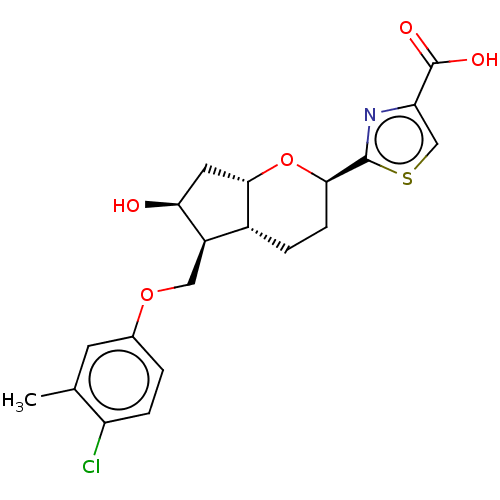 (CHEMBL3793515) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as intracellular Ca2+ level by fluorescence analysis | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50156529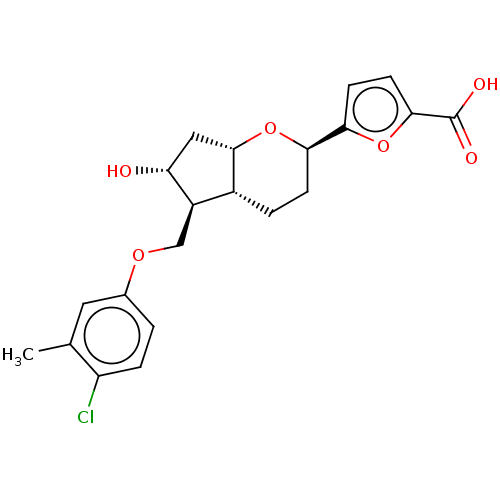 (CHEMBL3794226) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as intracellular Ca2+ level by fluorescence analysis | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50156528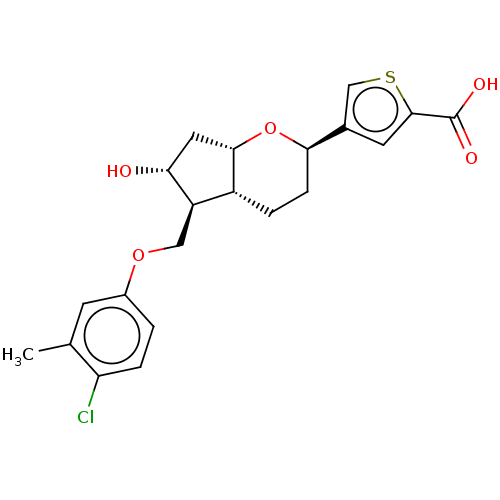 (CHEMBL3794185) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as intracellular Ca2+ level by fluorescence analysis | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50156491 (CHEMBL3792668) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in CHO cells assessed as intracellular Ca2+ level by fluorescence analysis | Bioorg Med Chem Lett 26: 2446-9 (2016) Article DOI: 10.1016/j.bmcl.2016.03.110 BindingDB Entry DOI: 10.7270/Q2H41T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50545404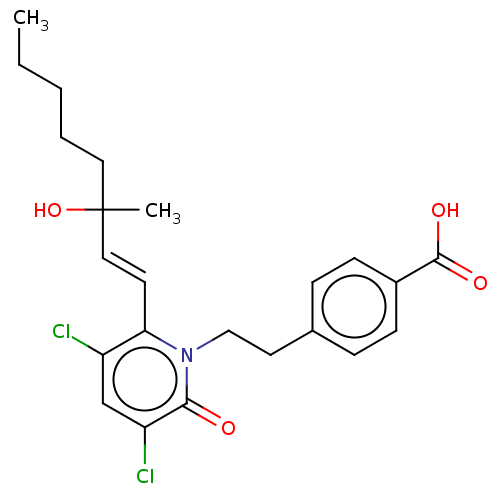 (CHEMBL4633041) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Inception Sciences Canada Inc. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in HEK293 cells by calcium-5 dye based FLIPR assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127104 BindingDB Entry DOI: 10.7270/Q29C7214 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50545403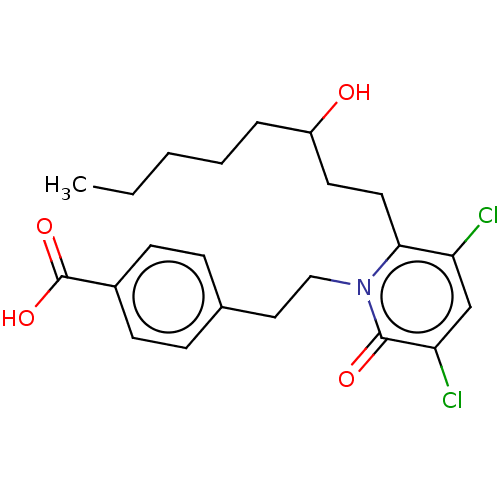 (CHEMBL4640635) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Inception Sciences Canada Inc. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in HEK293 cells by calcium-5 dye based FLIPR assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127104 BindingDB Entry DOI: 10.7270/Q29C7214 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50545402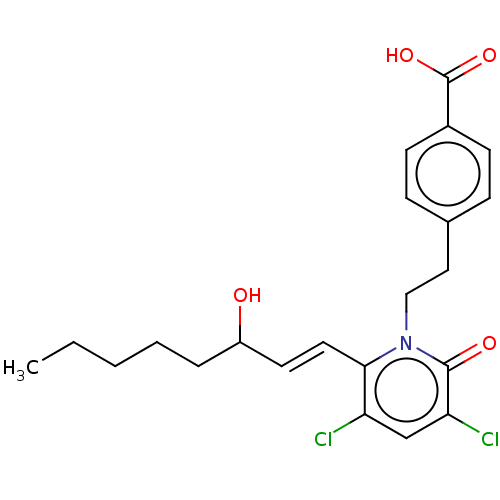 (CHEMBL4640391) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Inception Sciences Canada Inc. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in HEK293 cells by calcium-5 dye based FLIPR assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127104 BindingDB Entry DOI: 10.7270/Q29C7214 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50545399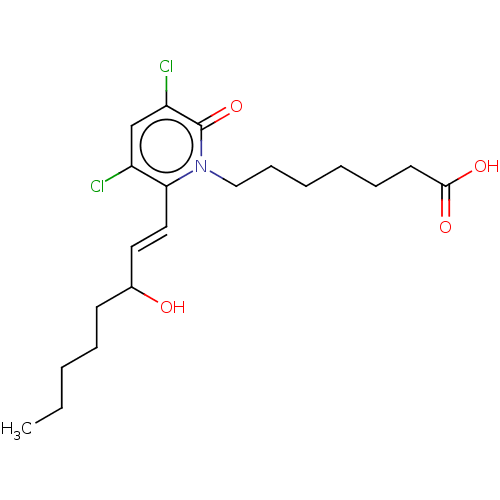 (CHEMBL4635212) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Inception Sciences Canada Inc. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in HEK293 cells by calcium-5 dye based FLIPR assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127104 BindingDB Entry DOI: 10.7270/Q29C7214 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50545400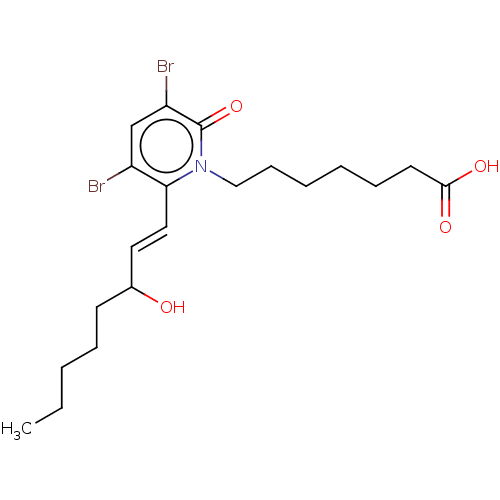 (CHEMBL4647921) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Inception Sciences Canada Inc. Curated by ChEMBL | Assay Description Agonist activity at human EP1 receptor expressed in HEK293 cells by calcium-5 dye based FLIPR assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127104 BindingDB Entry DOI: 10.7270/Q29C7214 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50085911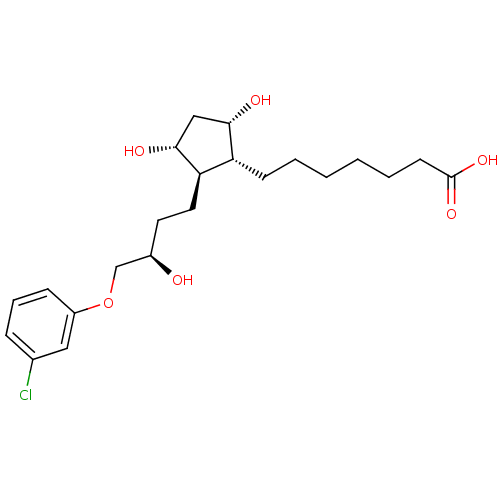 (7-{(1R,2R,3R,5S)-2-[(R)-4-(3-Chloro-phenoxy)-3-hyd...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Functional activity in RAT-1cells, transiently-transfected with human Prostaglandin E receptor EP1 (% of control ligand, 17-phi-PGE2=100%) | Bioorg Med Chem Lett 10: 1519-22 (2000) BindingDB Entry DOI: 10.7270/Q28W3CJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50090133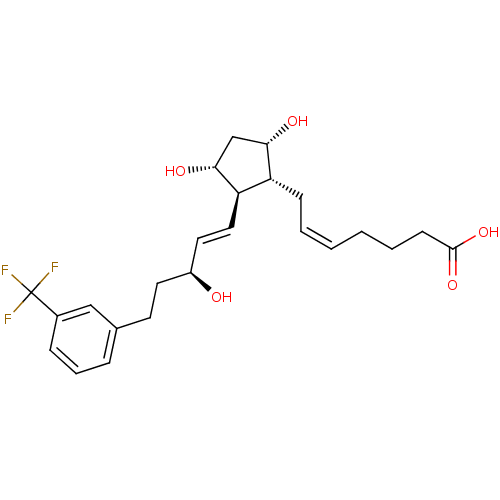 ((Z)-7-{(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(E)-(S)-3-hy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Functional activity in RAT-1cells, transiently-transfected with human Prostaglandin E receptor EP1 (% of control ligand, 17-phi-PGE2<50%) | Bioorg Med Chem Lett 10: 1519-22 (2000) BindingDB Entry DOI: 10.7270/Q28W3CJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50090137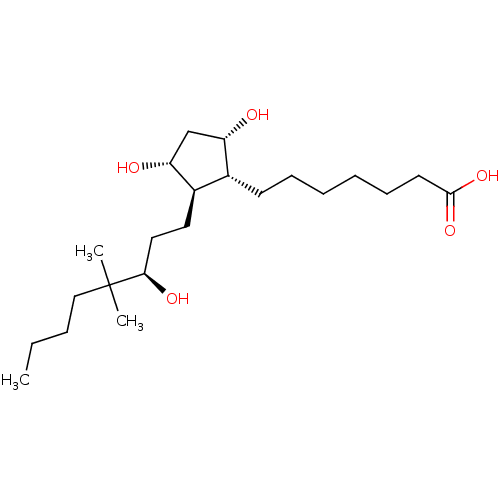 (7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-((R)-3-hydroxy-4,...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Functional activity in RAT-1cells, transiently-transfected with human Prostaglandin E receptor EP1 (% of control ligand, 17-phi-PGE2<20%) | Bioorg Med Chem Lett 10: 1519-22 (2000) BindingDB Entry DOI: 10.7270/Q28W3CJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50090140 (7-{(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(R)-3-hydroxy-5-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Functional activity in RAT-1cells, transiently-transfected with human Prostaglandin E receptor EP1 (% of control ligand, 17-phi-PGE2=100%) | Bioorg Med Chem Lett 10: 1519-22 (2000) BindingDB Entry DOI: 10.7270/Q28W3CJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||