

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549671 (CHEMBL4760558) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549681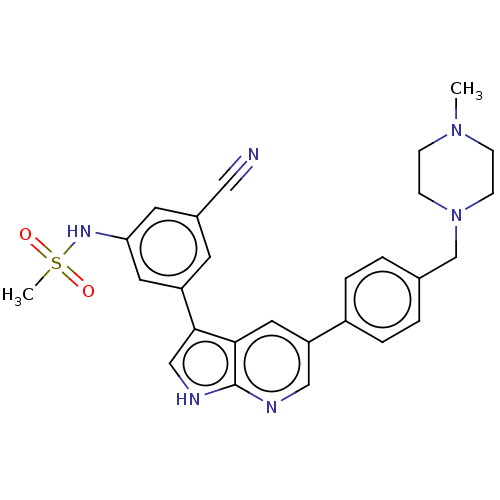 (CHEMBL4749291) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549672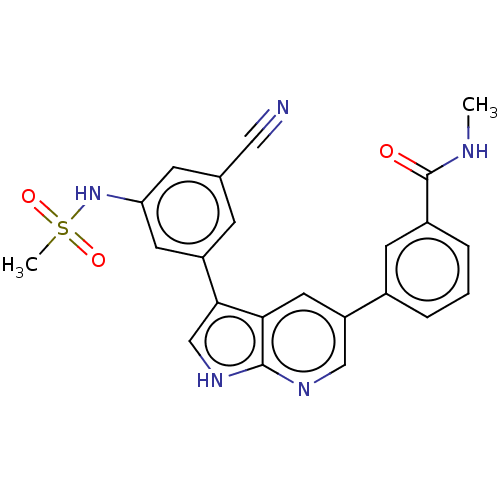 (CHEMBL4762464) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549680 (CHEMBL4759123) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549670 (CHEMBL4744045) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549674 (CHEMBL4751494) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549668 (CHEMBL4788405) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549669 (CHEMBL4788976) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549675 (CHEMBL4797452) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549673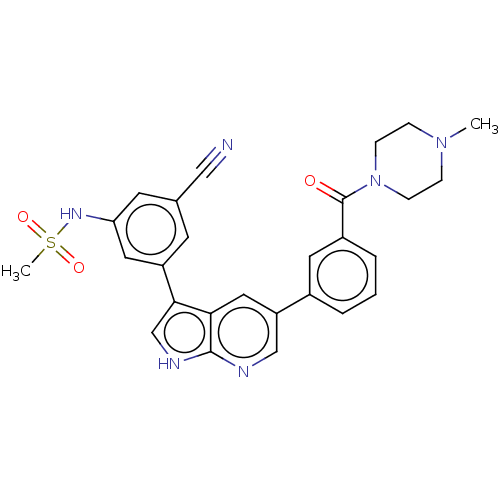 (CHEMBL4791330) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549664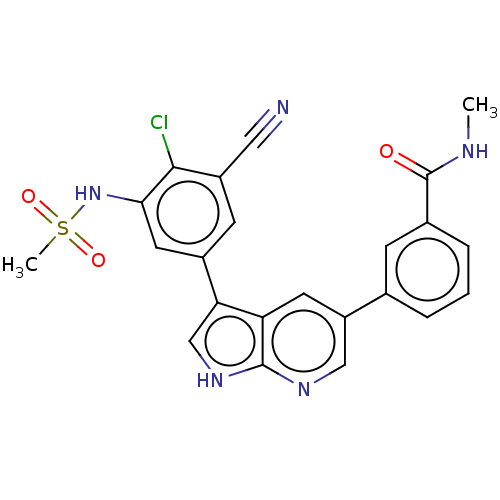 (CHEMBL4756005) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.314 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TNIK using RLGRDKYKTLRQIRQ as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549666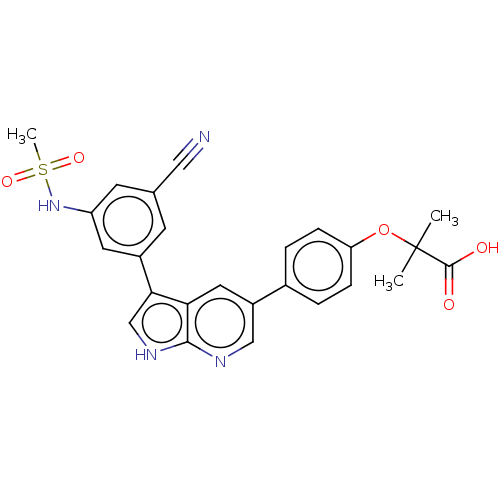 (CHEMBL4788688) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549679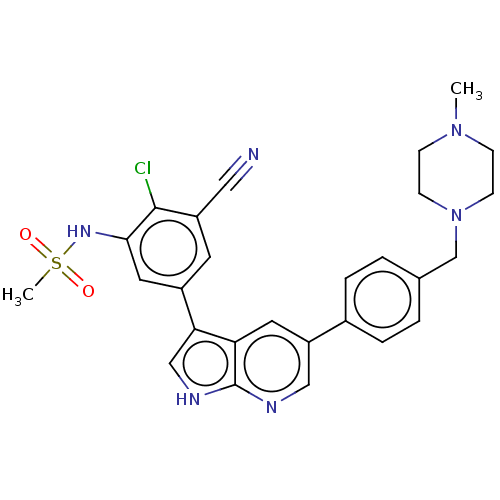 (CHEMBL4760435) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM420217 (US10485800, Cmpd ID FS | US10485800, Example 173) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | UniChem | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549667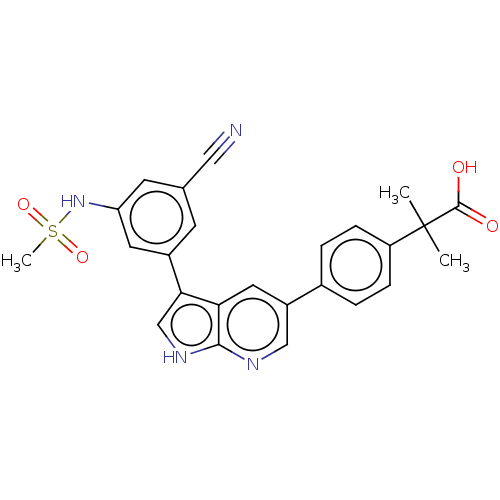 (CHEMBL4797770) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.551 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human TNIK using RLGRDKYKTLRQIRQ as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50550593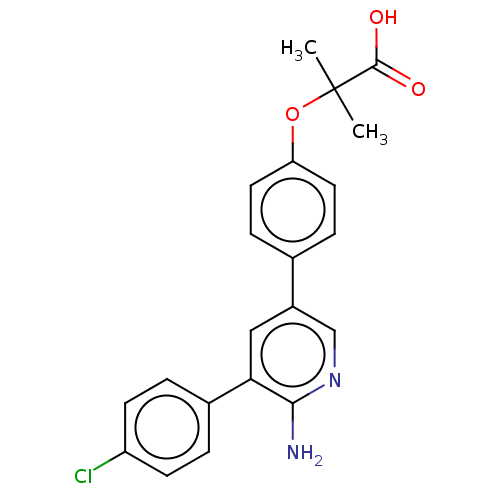 (CHEMBL4746566) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) in presence of 10 uM ATP | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b00152 BindingDB Entry DOI: 10.7270/Q2TH8RBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549663 (CHEMBL4780310) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549665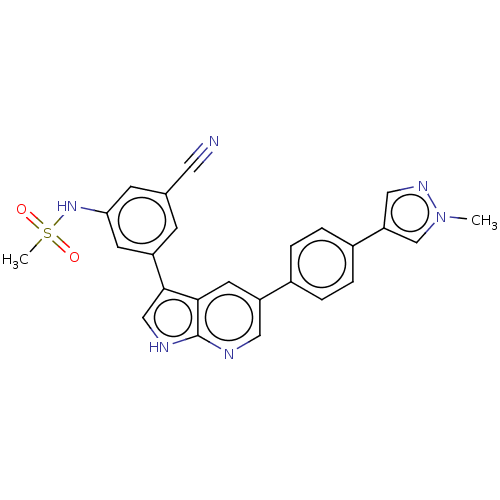 (CHEMBL4781043) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50549688 (CHEMBL4743105) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TNIK (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127749 BindingDB Entry DOI: 10.7270/Q2639TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579704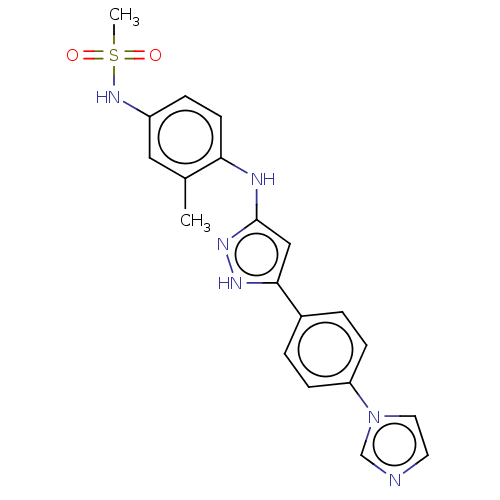 (N-(4-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579738 (N-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579718 (3-ethyl-4-((5-(4-iodophenyl)- 1H-pyrazol-3-yl)amin...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579709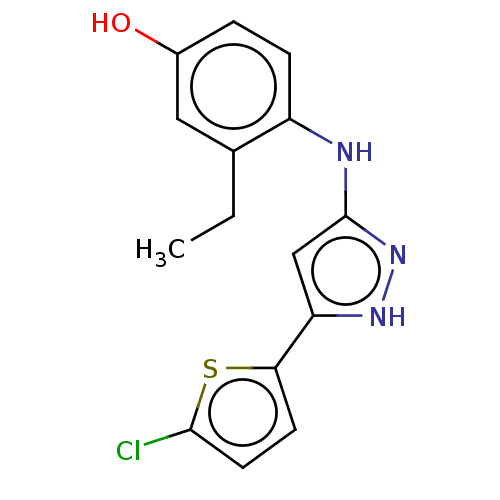 (4-((5-(5-chlorothiophen-2- yl)-1H-pyrazol-3-yl)ami...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571674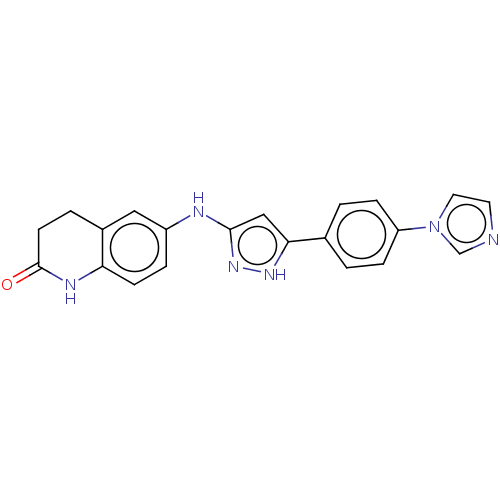 (6-((5-(4-(1H-imidazol-1- yl)phenyl)-1H-pyrazol-3- ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571620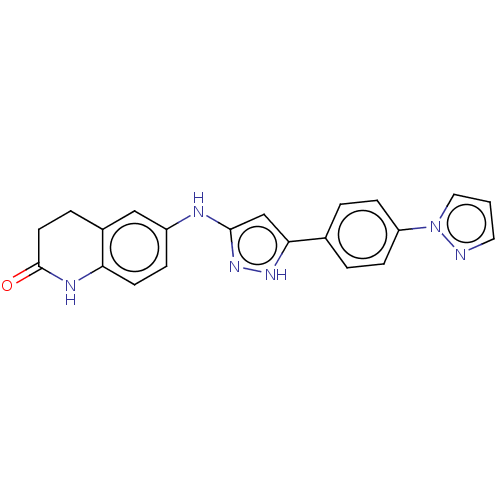 (6-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM571666 (5-((5-(3-fluoro-4- hydroxyphenyl)-1H- pyrazol-3- y...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11447469-20220920-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5C2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM613868 (5-(1-cyclopentyl-4-(4-fluorophenyl)-1H-imidazol-5-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | US Patent US11001589 (2021) BindingDB Entry DOI: 10.7270/Q2N30228 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM174187 (US9102637, 222) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
CARNA BIOSCIENCES, INC.; NATIONAL CANCER CENTER US Patent | Assay Description The kinase assays were conducted in a 20 μl volume using 384-well plates (Greiner). The reaction mixture consists of compound or vehicle (1% DMS... | US Patent US9102637 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50059889 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of TNIK (unknown origin) by ADP glo luminescent assay | Bioorg Med Chem Lett 23: 569-73 (2012) Article DOI: 10.1016/j.bmcl.2012.11.013 BindingDB Entry DOI: 10.7270/Q2G73G1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM174104 (US9102637, 139) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.5 | 25 |
CARNA BIOSCIENCES, INC.; NATIONAL CANCER CENTER US Patent | Assay Description The kinase assays were conducted in a 20 μl volume using 384-well plates (Greiner). The reaction mixture consists of compound or vehicle (1% DMS... | US Patent US9102637 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM613838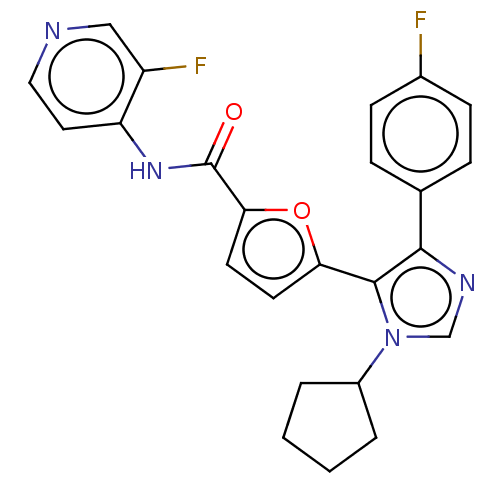 (5-(1-cyclopentyl-4-(4-fluorophenyl)-1H-imidazol-5-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.96 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | US Patent US11001589 (2021) BindingDB Entry DOI: 10.7270/Q2N30228 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM613838 (5-(1-cyclopentyl-4-(4-fluorophenyl)-1H-imidazol-5-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | US Patent US11001589 (2021) BindingDB Entry DOI: 10.7270/Q2N30228 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579739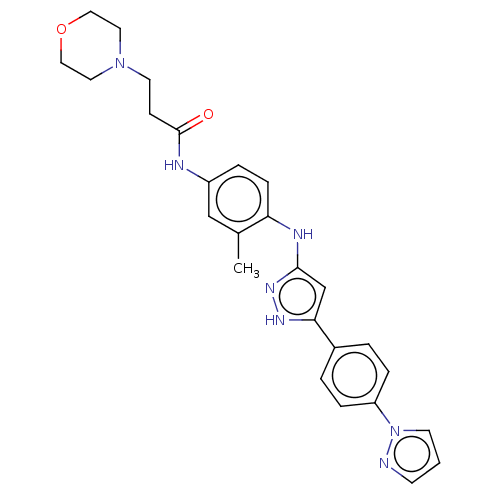 (N-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM174202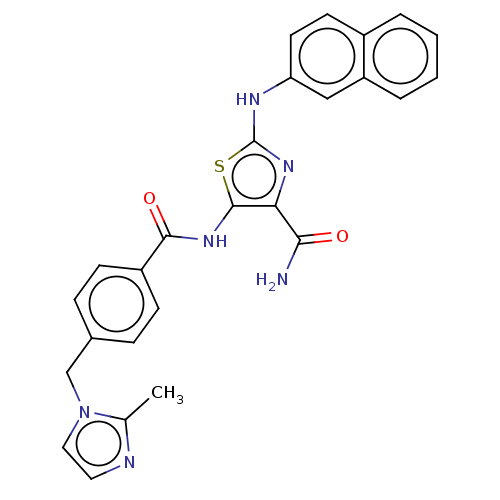 (US9102637, 237) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
CARNA BIOSCIENCES, INC.; NATIONAL CANCER CENTER US Patent | Assay Description The kinase assays were conducted in a 20 μl volume using 384-well plates (Greiner). The reaction mixture consists of compound or vehicle (1% DMS... | US Patent US9102637 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM613765 (5-(1-cyclopentyl-4-(4-fluorophenyl)-1H-imidazol-5-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.33 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | US Patent US11001589 (2021) BindingDB Entry DOI: 10.7270/Q2N30228 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM174160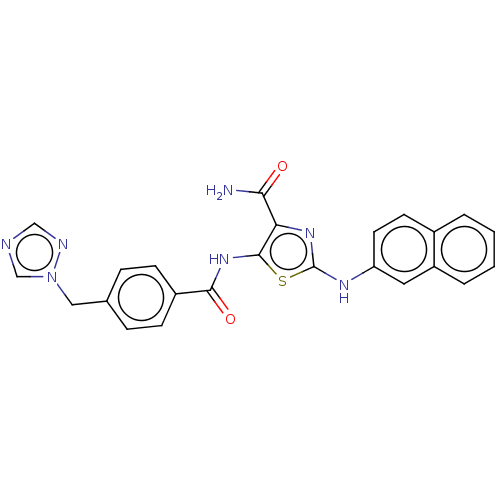 (US9102637, 195) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
CARNA BIOSCIENCES, INC.; NATIONAL CANCER CENTER US Patent | Assay Description The kinase assays were conducted in a 20 μl volume using 384-well plates (Greiner). The reaction mixture consists of compound or vehicle (1% DMS... | US Patent US9102637 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM174186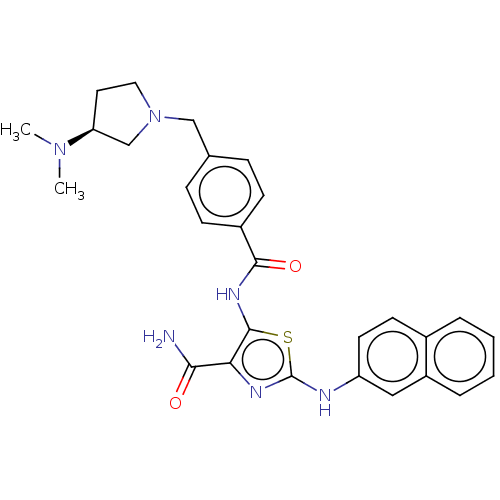 (US9102637, 221) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.5 | 25 |
CARNA BIOSCIENCES, INC.; NATIONAL CANCER CENTER US Patent | Assay Description The kinase assays were conducted in a 20 μl volume using 384-well plates (Greiner). The reaction mixture consists of compound or vehicle (1% DMS... | US Patent US9102637 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM174150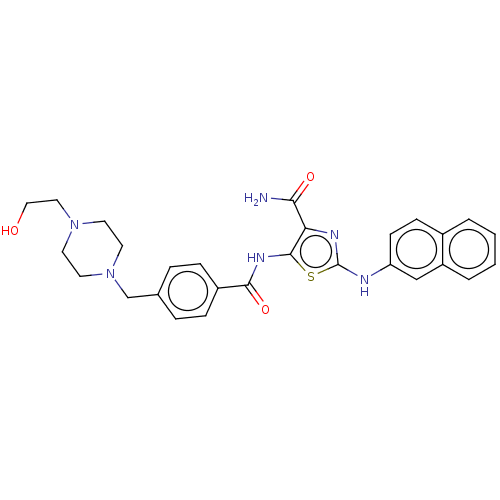 (US9102637, 185) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | 25 |
CARNA BIOSCIENCES, INC.; NATIONAL CANCER CENTER US Patent | Assay Description The kinase assays were conducted in a 20 μl volume using 384-well plates (Greiner). The reaction mixture consists of compound or vehicle (1% DMS... | US Patent US9102637 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM613877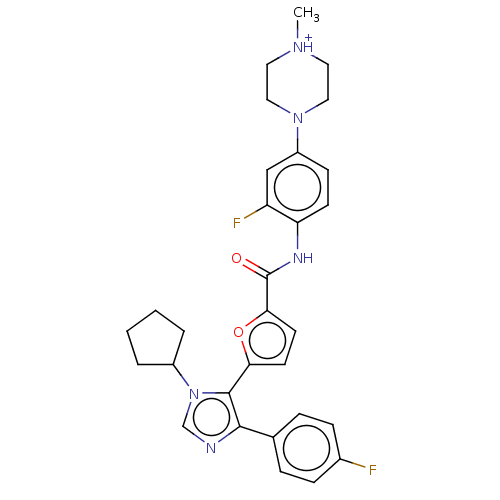 (4-(4-(5-(1-cyclopentyl-4-(4-fluorophenyl)-1H-imida...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | US Patent US11001589 (2021) BindingDB Entry DOI: 10.7270/Q2N30228 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM174196 (US9102637, 231) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.5 | 25 |
CARNA BIOSCIENCES, INC.; NATIONAL CANCER CENTER US Patent | Assay Description The kinase assays were conducted in a 20 μl volume using 384-well plates (Greiner). The reaction mixture consists of compound or vehicle (1% DMS... | US Patent US9102637 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM174173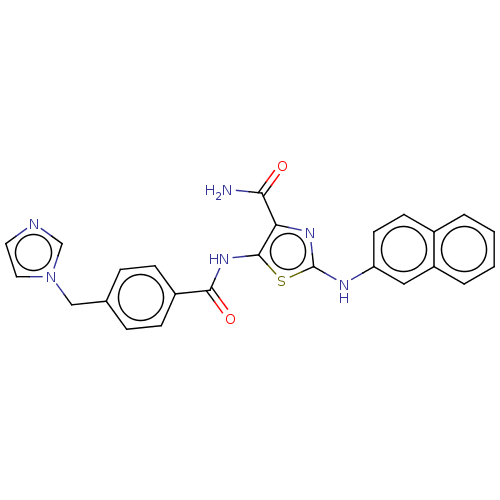 (US9102637, 208) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
CARNA BIOSCIENCES, INC.; NATIONAL CANCER CENTER US Patent | Assay Description The kinase assays were conducted in a 20 μl volume using 384-well plates (Greiner). The reaction mixture consists of compound or vehicle (1% DMS... | US Patent US9102637 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM50424547 (CHEMBL2312144) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of TNIK (unknown origin) by ADP glo luminescent assay | Bioorg Med Chem Lett 23: 569-73 (2012) Article DOI: 10.1016/j.bmcl.2012.11.013 BindingDB Entry DOI: 10.7270/Q2G73G1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM174215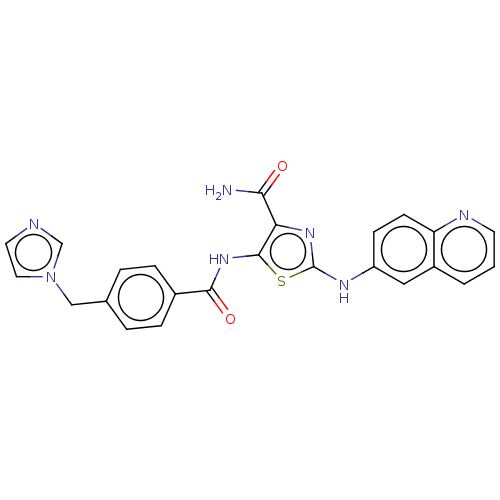 (US9102637, 250) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
CARNA BIOSCIENCES, INC.; NATIONAL CANCER CENTER US Patent | Assay Description The kinase assays were conducted in a 20 μl volume using 384-well plates (Greiner). The reaction mixture consists of compound or vehicle (1% DMS... | US Patent US9102637 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579689 (N-(4-((5-(4-hydroxyphenyl)- 1H-pyrazol-3-yl)amino)...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM579743 (N-(4-((5-(4-(1H-pyrazol-1- yl)phenyl)-1H-pyrazol-3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory properties of compounds were evaluated with TNIK kinase enzyme system and luminescent ADP-GloFigure US11485711-20221101-P00001 Kinase ... | Citation and Details BindingDB Entry DOI: 10.7270/Q21R6VC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM174185 (US9102637, 220) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
CARNA BIOSCIENCES, INC.; NATIONAL CANCER CENTER US Patent | Assay Description The kinase assays were conducted in a 20 μl volume using 384-well plates (Greiner). The reaction mixture consists of compound or vehicle (1% DMS... | US Patent US9102637 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM608272 (5-(1-cyclopentyl-4-(4-fluorophenyl)-1H-imidazol-5-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.07 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | US Patent US11001589 (2021) BindingDB Entry DOI: 10.7270/Q2N30228 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TRAF2 and NCK-interacting protein kinase (Homo sapiens (Human)) | BDBM174203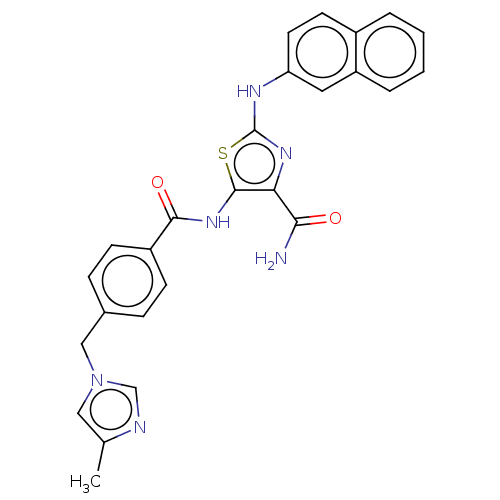 (US9102637, 238) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
CARNA BIOSCIENCES, INC.; NATIONAL CANCER CENTER US Patent | Assay Description The kinase assays were conducted in a 20 μl volume using 384-well plates (Greiner). The reaction mixture consists of compound or vehicle (1% DMS... | US Patent US9102637 (2015) BindingDB Entry DOI: 10.7270/Q2KS6Q9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 740 total ) | Next | Last >> |