 Found 49 hits Enz. Inhib. hit(s) with all data for entry = 4784
Found 49 hits Enz. Inhib. hit(s) with all data for entry = 4784 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82063
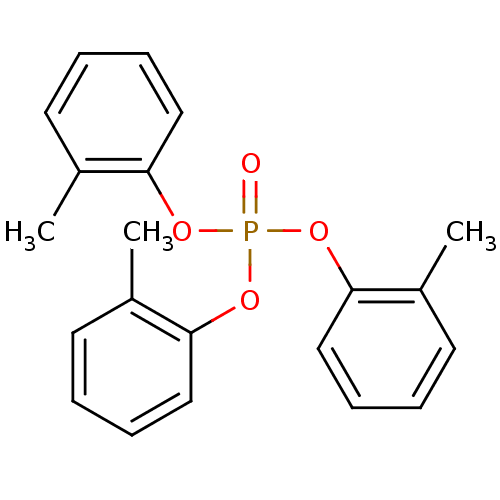
(CAS_78-30-8 | TCP | TRI-O-CRESYL PHOSPHATE)Show InChI InChI=1S/C21H21O4P/c1-16-10-4-7-13-19(16)23-26(22,24-20-14-8-5-11-17(20)2)25-21-15-9-6-12-18(21)3/h4-15H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82063

(CAS_78-30-8 | TCP | TRI-O-CRESYL PHOSPHATE)Show InChI InChI=1S/C21H21O4P/c1-16-10-4-7-13-19(16)23-26(22,24-20-14-8-5-11-17(20)2)25-21-15-9-6-12-18(21)3/h4-15H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001028
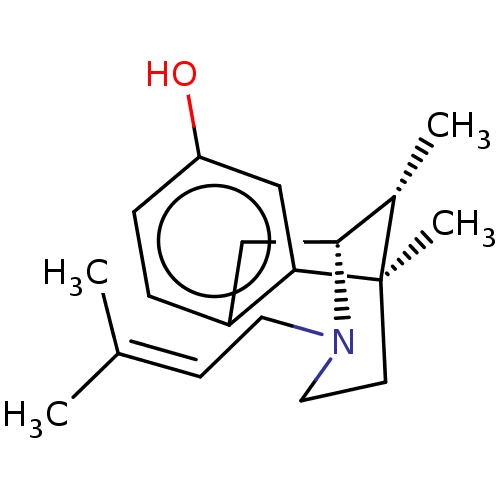
((+)-PENTAZOCINE | (-)-pentazocine | (2R,6R,11R)-6,...)Show SMILES [#6]-[#6@H]1-[#6@H]-2-[#6]-c3ccc(-[#8])cc3[C@]1([#6])[#6]-[#6]-[#7]-2-[#6]\[#6]=[#6](\[#6])-[#6] |r,TLB:16:15:1:3.4.10| Show InChI InChI=1S/C19H27NO/c1-13(2)7-9-20-10-8-19(4)14(3)18(20)11-15-5-6-16(21)12-17(15)19/h5-7,12,14,18,21H,8-11H2,1-4H3/t14-,18+,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82062
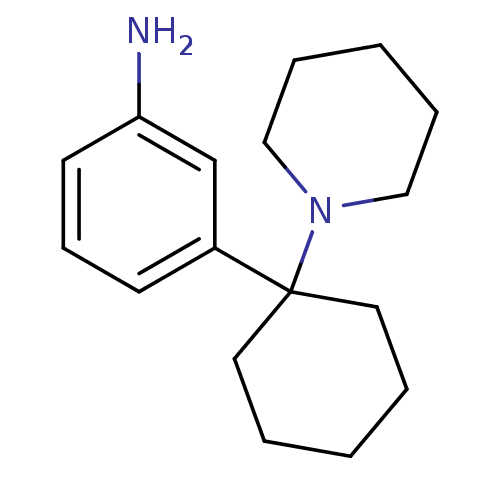
(PCP,m-NH2)Show InChI InChI=1S/C17H26N2/c18-16-9-7-8-15(14-16)17(10-3-1-4-11-17)19-12-5-2-6-13-19/h7-9,14H,1-6,10-13,18H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82062

(PCP,m-NH2)Show InChI InChI=1S/C17H26N2/c18-16-9-7-8-15(14-16)17(10-3-1-4-11-17)19-12-5-2-6-13-19/h7-9,14H,1-6,10-13,18H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001028

((+)-PENTAZOCINE | (-)-pentazocine | (2R,6R,11R)-6,...)Show SMILES [#6]-[#6@H]1-[#6@H]-2-[#6]-c3ccc(-[#8])cc3[C@]1([#6])[#6]-[#6]-[#7]-2-[#6]\[#6]=[#6](\[#6])-[#6] |r,TLB:16:15:1:3.4.10| Show InChI InChI=1S/C19H27NO/c1-13(2)7-9-20-10-8-19(4)14(3)18(20)11-15-5-6-16(21)12-17(15)19/h5-7,12,14,18,21H,8-11H2,1-4H3/t14-,18+,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50002051
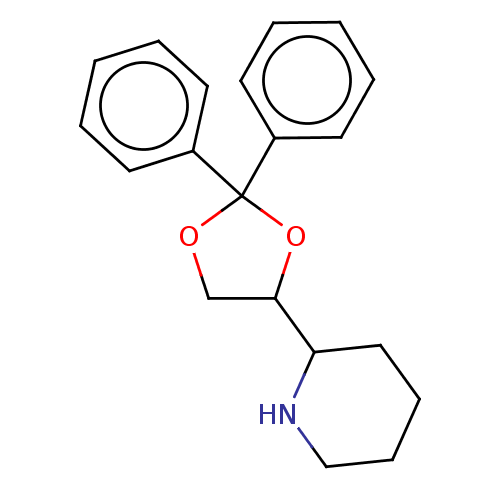
((+/-)-(2RS)-2-[(4RS)-2,2-Diphenyl-1,3-dioxolan-4-y...)Show InChI InChI=1S/C20H23NO2/c1-3-9-16(10-4-1)20(17-11-5-2-6-12-17)22-15-19(23-20)18-13-7-8-14-21-18/h1-6,9-12,18-19,21H,7-8,13-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50010617

((-)-3-(1-Propyl-piperidin-3-yl)-phenol | (S)-3-(1-...)Show InChI InChI=1S/C14H21NO/c1-2-8-15-9-4-6-13(11-15)12-5-3-7-14(16)10-12/h3,5,7,10,13,16H,2,4,6,8-9,11H2,1H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50002051

((+/-)-(2RS)-2-[(4RS)-2,2-Diphenyl-1,3-dioxolan-4-y...)Show InChI InChI=1S/C20H23NO2/c1-3-9-16(10-4-1)20(17-11-5-2-6-12-17)22-15-19(23-20)18-13-7-8-14-21-18/h1-6,9-12,18-19,21H,7-8,13-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM83449
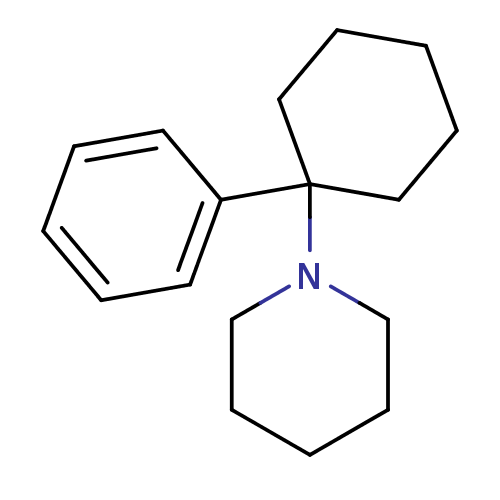
(1-(1-phenylcyclohexyl)piperidine;hydrochloride | M...)Show InChI InChI=1S/C17H25N/c1-4-10-16(11-5-1)17(12-6-2-7-13-17)18-14-8-3-9-15-18/h1,4-5,10-11H,2-3,6-9,12-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50010617

((-)-3-(1-Propyl-piperidin-3-yl)-phenol | (S)-3-(1-...)Show InChI InChI=1S/C14H21NO/c1-2-8-15-9-4-6-13(11-15)12-5-3-7-14(16)10-12/h3,5,7,10,13,16H,2,4,6,8-9,11H2,1H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001031

(3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2,6-me...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CC=C |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C17H23NO/c1-4-8-18-9-7-17(3)12(2)16(18)10-13-5-6-14(19)11-15(13)17/h4-6,11-12,16,19H,1,7-10H2,2-3H3/t12-,16+,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM83449

(1-(1-phenylcyclohexyl)piperidine;hydrochloride | M...)Show InChI InChI=1S/C17H25N/c1-4-10-16(11-5-1)17(12-6-2-7-13-17)18-14-8-3-9-15-18/h1,4-5,10-11H,2-3,6-9,12-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001031

(3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2,6-me...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CC=C |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C17H23NO/c1-4-8-18-9-7-17(3)12(2)16(18)10-13-5-6-14(19)11-15(13)17/h4-6,11-12,16,19H,1,7-10H2,2-3H3/t12-,16+,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82063

(CAS_78-30-8 | TCP | TRI-O-CRESYL PHOSPHATE)Show InChI InChI=1S/C21H21O4P/c1-16-10-4-7-13-19(16)23-26(22,24-20-14-8-5-11-17(20)2)25-21-15-9-6-12-18(21)3/h4-15H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001028

((+)-PENTAZOCINE | (-)-pentazocine | (2R,6R,11R)-6,...)Show SMILES [#6]-[#6@H]1-[#6@H]-2-[#6]-c3ccc(-[#8])cc3[C@]1([#6])[#6]-[#6]-[#7]-2-[#6]\[#6]=[#6](\[#6])-[#6] |r,TLB:16:15:1:3.4.10| Show InChI InChI=1S/C19H27NO/c1-13(2)7-9-20-10-8-19(4)14(3)18(20)11-15-5-6-16(21)12-17(15)19/h5-7,12,14,18,21H,8-11H2,1-4H3/t14-,18+,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM83449

(1-(1-phenylcyclohexyl)piperidine;hydrochloride | M...)Show InChI InChI=1S/C17H25N/c1-4-10-16(11-5-1)17(12-6-2-7-13-17)18-14-8-3-9-15-18/h1,4-5,10-11H,2-3,6-9,12-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82062

(PCP,m-NH2)Show InChI InChI=1S/C17H26N2/c18-16-9-7-8-15(14-16)17(10-3-1-4-11-17)19-12-5-2-6-13-19/h7-9,14H,1-6,10-13,18H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001031

(3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2,6-me...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CC=C |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C17H23NO/c1-4-8-18-9-7-17(3)12(2)16(18)10-13-5-6-14(19)11-15(13)17/h4-6,11-12,16,19H,1,7-10H2,2-3H3/t12-,16+,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001031

(3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2,6-me...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CC=C |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C17H23NO/c1-4-8-18-9-7-17(3)12(2)16(18)10-13-5-6-14(19)11-15(13)17/h4-6,11-12,16,19H,1,7-10H2,2-3H3/t12-,16+,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 375 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001031

(3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2,6-me...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CC=C |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C17H23NO/c1-4-8-18-9-7-17(3)12(2)16(18)10-13-5-6-14(19)11-15(13)17/h4-6,11-12,16,19H,1,7-10H2,2-3H3/t12-,16+,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001031

(3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2,6-me...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CC=C |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C17H23NO/c1-4-8-18-9-7-17(3)12(2)16(18)10-13-5-6-14(19)11-15(13)17/h4-6,11-12,16,19H,1,7-10H2,2-3H3/t12-,16+,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50002051

((+/-)-(2RS)-2-[(4RS)-2,2-Diphenyl-1,3-dioxolan-4-y...)Show InChI InChI=1S/C20H23NO2/c1-3-9-16(10-4-1)20(17-11-5-2-6-12-17)22-15-19(23-20)18-13-7-8-14-21-18/h1-6,9-12,18-19,21H,7-8,13-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 569 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM83449

(1-(1-phenylcyclohexyl)piperidine;hydrochloride | M...)Show InChI InChI=1S/C17H25N/c1-4-10-16(11-5-1)17(12-6-2-7-13-17)18-14-8-3-9-15-18/h1,4-5,10-11H,2-3,6-9,12-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 625 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001031

(3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2,6-me...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CC=C |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C17H23NO/c1-4-8-18-9-7-17(3)12(2)16(18)10-13-5-6-14(19)11-15(13)17/h4-6,11-12,16,19H,1,7-10H2,2-3H3/t12-,16+,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM83449

(1-(1-phenylcyclohexyl)piperidine;hydrochloride | M...)Show InChI InChI=1S/C17H25N/c1-4-10-16(11-5-1)17(12-6-2-7-13-17)18-14-8-3-9-15-18/h1,4-5,10-11H,2-3,6-9,12-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 758 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001031

(3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2,6-me...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CC=C |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C17H23NO/c1-4-8-18-9-7-17(3)12(2)16(18)10-13-5-6-14(19)11-15(13)17/h4-6,11-12,16,19H,1,7-10H2,2-3H3/t12-,16+,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 821 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001031

(3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2,6-me...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CC=C |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C17H23NO/c1-4-8-18-9-7-17(3)12(2)16(18)10-13-5-6-14(19)11-15(13)17/h4-6,11-12,16,19H,1,7-10H2,2-3H3/t12-,16+,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 895 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50002051

((+/-)-(2RS)-2-[(4RS)-2,2-Diphenyl-1,3-dioxolan-4-y...)Show InChI InChI=1S/C20H23NO2/c1-3-9-16(10-4-1)20(17-11-5-2-6-12-17)22-15-19(23-20)18-13-7-8-14-21-18/h1-6,9-12,18-19,21H,7-8,13-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001031

(3-Allyl-6,11-dimethyl-1,2,3,4,5,6-hexahydro-2,6-me...)Show SMILES C[C@H]1[C@H]2Cc3ccc(O)cc3[C@@]1(C)CCN2CC=C |TLB:16:15:1:10.4.3| Show InChI InChI=1S/C17H23NO/c1-4-8-18-9-7-17(3)12(2)16(18)10-13-5-6-14(19)11-15(13)17/h4-6,11-12,16,19H,1,7-10H2,2-3H3/t12-,16+,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001028

((+)-PENTAZOCINE | (-)-pentazocine | (2R,6R,11R)-6,...)Show SMILES [#6]-[#6@H]1-[#6@H]-2-[#6]-c3ccc(-[#8])cc3[C@]1([#6])[#6]-[#6]-[#7]-2-[#6]\[#6]=[#6](\[#6])-[#6] |r,TLB:16:15:1:3.4.10| Show InChI InChI=1S/C19H27NO/c1-13(2)7-9-20-10-8-19(4)14(3)18(20)11-15-5-6-16(21)12-17(15)19/h5-7,12,14,18,21H,8-11H2,1-4H3/t14-,18+,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82062

(PCP,m-NH2)Show InChI InChI=1S/C17H26N2/c18-16-9-7-8-15(14-16)17(10-3-1-4-11-17)19-12-5-2-6-13-19/h7-9,14H,1-6,10-13,18H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50002051

((+/-)-(2RS)-2-[(4RS)-2,2-Diphenyl-1,3-dioxolan-4-y...)Show InChI InChI=1S/C20H23NO2/c1-3-9-16(10-4-1)20(17-11-5-2-6-12-17)22-15-19(23-20)18-13-7-8-14-21-18/h1-6,9-12,18-19,21H,7-8,13-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82003
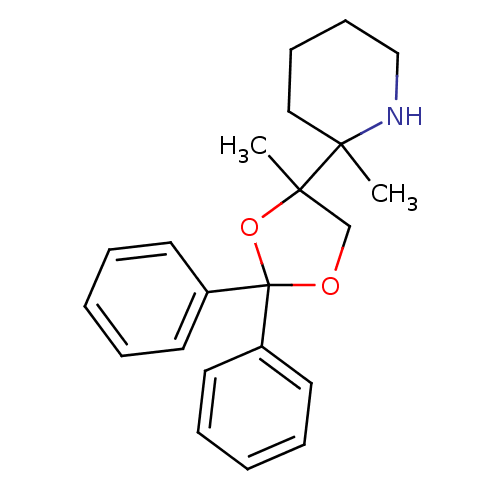
(CAS_4792-18-1 | Levoxadrol | NSC_31786)Show InChI InChI=1S/C22H27NO2/c1-20(15-9-10-16-23-20)21(2)17-24-22(25-21,18-11-5-3-6-12-18)19-13-7-4-8-14-19/h3-8,11-14,23H,9-10,15-17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82003

(CAS_4792-18-1 | Levoxadrol | NSC_31786)Show InChI InChI=1S/C22H27NO2/c1-20(15-9-10-16-23-20)21(2)17-24-22(25-21,18-11-5-3-6-12-18)19-13-7-4-8-14-19/h3-8,11-14,23H,9-10,15-17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50001028

((+)-PENTAZOCINE | (-)-pentazocine | (2R,6R,11R)-6,...)Show SMILES [#6]-[#6@H]1-[#6@H]-2-[#6]-c3ccc(-[#8])cc3[C@]1([#6])[#6]-[#6]-[#7]-2-[#6]\[#6]=[#6](\[#6])-[#6] |r,TLB:16:15:1:3.4.10| Show InChI InChI=1S/C19H27NO/c1-13(2)7-9-20-10-8-19(4)14(3)18(20)11-15-5-6-16(21)12-17(15)19/h5-7,12,14,18,21H,8-11H2,1-4H3/t14-,18+,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82062

(PCP,m-NH2)Show InChI InChI=1S/C17H26N2/c18-16-9-7-8-15(14-16)17(10-3-1-4-11-17)19-12-5-2-6-13-19/h7-9,14H,1-6,10-13,18H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82063

(CAS_78-30-8 | TCP | TRI-O-CRESYL PHOSPHATE)Show InChI InChI=1S/C21H21O4P/c1-16-10-4-7-13-19(16)23-26(22,24-20-14-8-5-11-17(20)2)25-21-15-9-6-12-18(21)3/h4-15H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82003

(CAS_4792-18-1 | Levoxadrol | NSC_31786)Show InChI InChI=1S/C22H27NO2/c1-20(15-9-10-16-23-20)21(2)17-24-22(25-21,18-11-5-3-6-12-18)19-13-7-4-8-14-19/h3-8,11-14,23H,9-10,15-17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 3.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82063

(CAS_78-30-8 | TCP | TRI-O-CRESYL PHOSPHATE)Show InChI InChI=1S/C21H21O4P/c1-16-10-4-7-13-19(16)23-26(22,24-20-14-8-5-11-17(20)2)25-21-15-9-6-12-18(21)3/h4-15H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82003

(CAS_4792-18-1 | Levoxadrol | NSC_31786)Show InChI InChI=1S/C22H27NO2/c1-20(15-9-10-16-23-20)21(2)17-24-22(25-21,18-11-5-3-6-12-18)19-13-7-4-8-14-19/h3-8,11-14,23H,9-10,15-17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50010617

((-)-3-(1-Propyl-piperidin-3-yl)-phenol | (S)-3-(1-...)Show InChI InChI=1S/C14H21NO/c1-2-8-15-9-4-6-13(11-15)12-5-3-7-14(16)10-12/h3,5,7,10,13,16H,2,4,6,8-9,11H2,1H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM50010617

((-)-3-(1-Propyl-piperidin-3-yl)-phenol | (S)-3-(1-...)Show InChI InChI=1S/C14H21NO/c1-2-8-15-9-4-6-13(11-15)12-5-3-7-14(16)10-12/h3,5,7,10,13,16H,2,4,6,8-9,11H2,1H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM82003

(CAS_4792-18-1 | Levoxadrol | NSC_31786)Show InChI InChI=1S/C22H27NO2/c1-20(15-9-10-16-23-20)21(2)17-24-22(25-21,18-11-5-3-6-12-18)19-13-7-4-8-14-19/h3-8,11-14,23H,9-10,15-17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 238: 739-48 (1986)
BindingDB Entry DOI: 10.7270/Q2C24TX8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data