 Found 7 hits Enz. Inhib. hit(s) with all data for entry = 5060
Found 7 hits Enz. Inhib. hit(s) with all data for entry = 5060 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50292411
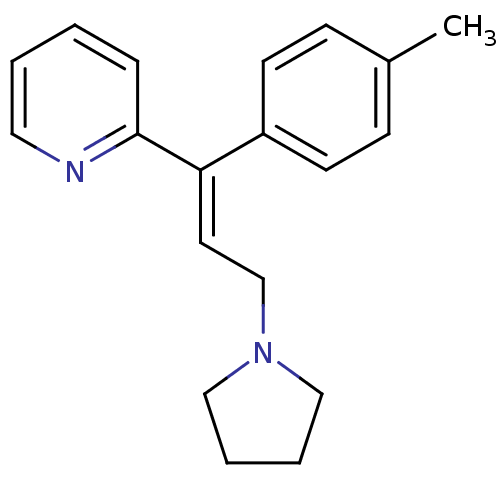
((E)-2-(3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl)py...)Show InChI InChI=1S/C19H22N2/c1-16-7-9-17(10-8-16)18(19-6-2-3-12-20-19)11-15-21-13-4-5-14-21/h2-3,6-12H,4-5,13-15H2,1H3/b18-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM35938
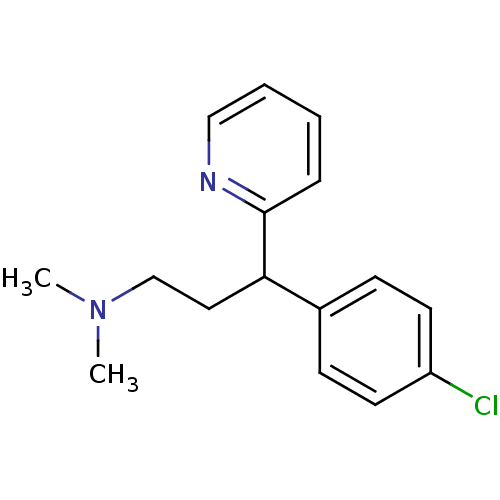
(1-(p-chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpro...)Show InChI InChI=1S/C16H19ClN2/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13/h3-9,11,15H,10,12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM85029
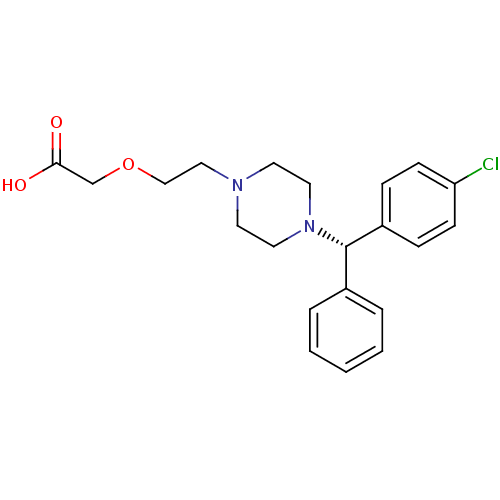
(Cetirizine (+) isomer)Show SMILES OC(=O)COCCN1CCN(CC1)[C@@H](c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H25ClN2O3/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26/h1-9,21H,10-16H2,(H,25,26)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22879
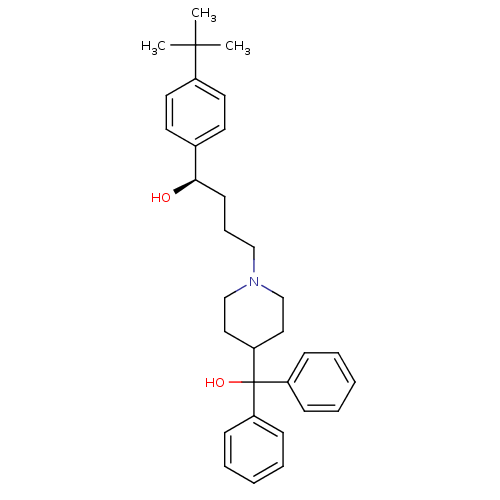
((1R)-1-(4-tert-butylphenyl)-4-[4-(hydroxydiphenylm...)Show SMILES CC(C)(C)c1ccc(cc1)[C@H](O)CCCN1CCC(CC1)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567

(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM85030

(Cetirizine (-) isomer)Show SMILES OC(=O)COCCN1CCN(CC1)[C@H](c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H25ClN2O3/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26/h1-9,21H,10-16H2,(H,25,26)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Libre de Bruxelles
Curated by PDSP Ki Database
| |
J Recept Signal Transduct Res 15: 91-102 (1995)
Article DOI: 10.3109/10799899509045210
BindingDB Entry DOI: 10.7270/Q2PZ57BR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data