

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50024317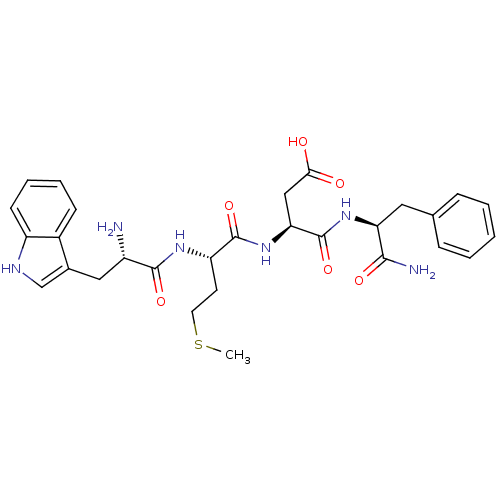 (3-{2-[2-Amino-3-(1H-indol-3-yl)-propionylamino]-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancr... | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50024317 (3-{2-[2-Amino-3-(1H-indol-3-yl)-propionylamino]-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type B receptor in the mouse cer... | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50283251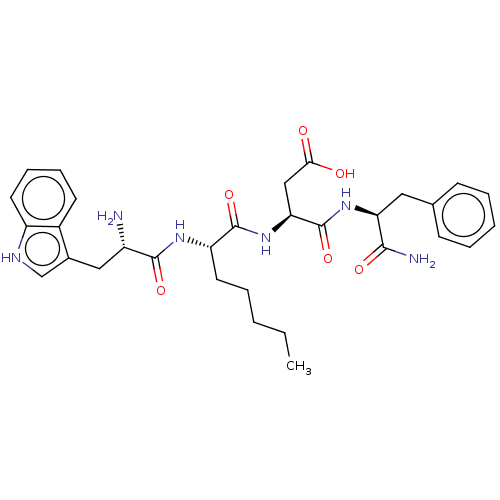 (3-{(S)-2-[2-Amino-3-(1H-indol-3-yl)-propionylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type B receptor in the mouse cer... | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50283251 (3-{(S)-2-[2-Amino-3-(1H-indol-3-yl)-propionylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancr... | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50450087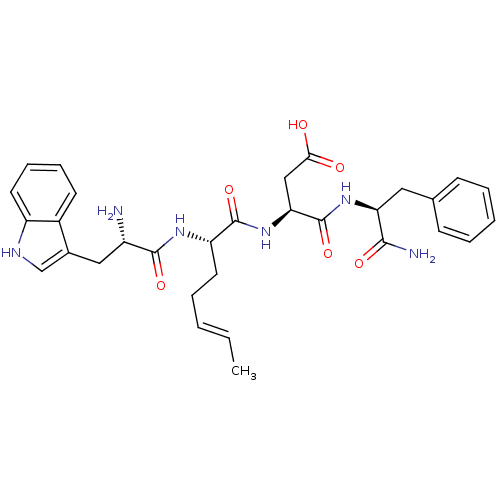 (CHEMBL2369769) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type B receptor in the mouse cer... | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50283254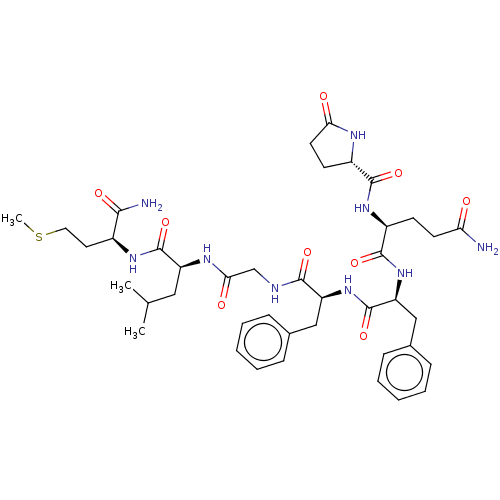 ((S)-2-[(5-Oxo-pyrrolidine-2-carbonyl)-amino]-penta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of. [125I]-Bolton-Hunter Substance P to NK-1 receptors in the guinea pig cerebral... | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50283257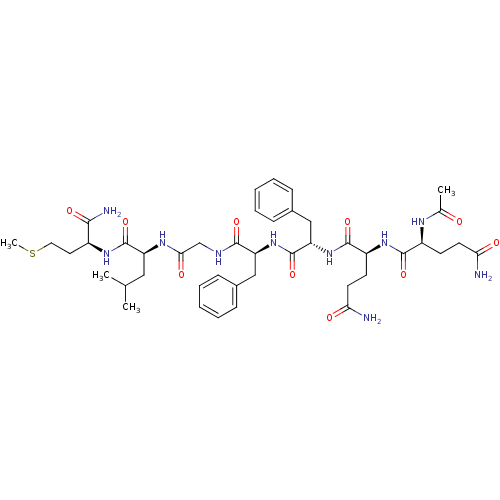 ((S)-2-[2-((S)-2-{(S)-2-[(S)-2-((S)-2-Acetylamino-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of. [125I]-Bolton-Hunter Substance P to NK-1 receptors in the guinea pig cerebral... | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50283253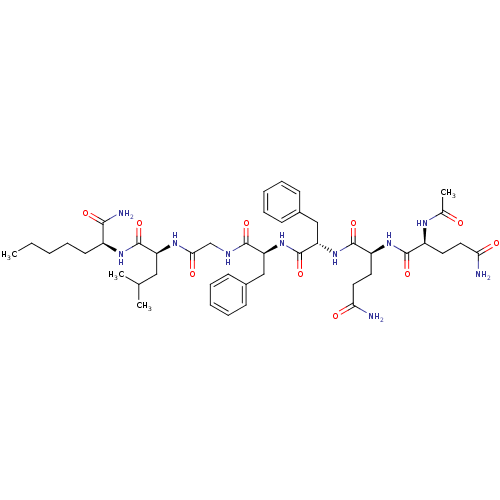 ((S)-2-[2-((S)-2-{(S)-2-[(S)-2-((S)-2-Acetylamino-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of. [125I]-Bolton-Hunter Substance P to NK-1 receptors in the guinea pig cerebral... | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50283252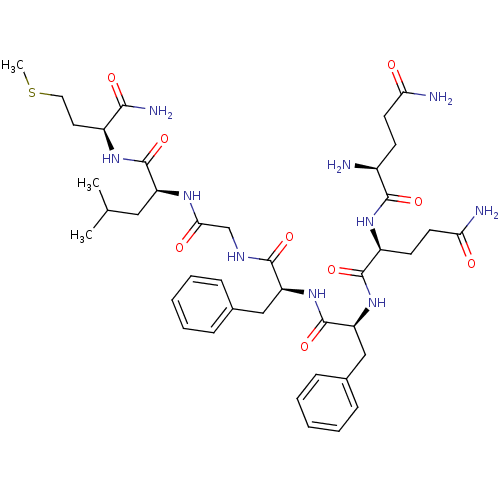 ((S)-2-[2-((S)-2-{(S)-2-[(S)-2-((S)-2-Amino-4-carba...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of. [125I]-Bolton-Hunter Substance P to NK-1 receptors in the guinea pig cerebral... | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50283256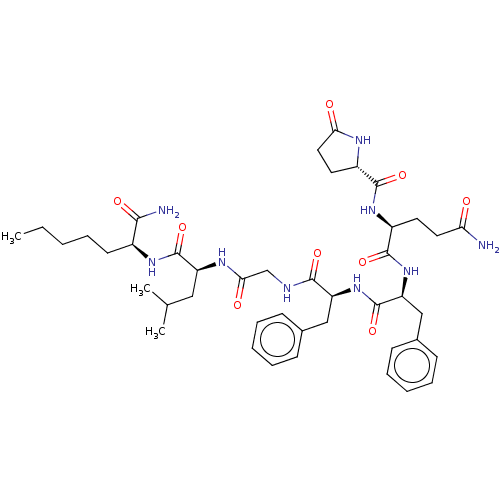 ((S)-2-[(5-Oxo-pyrrolidine-2-carbonyl)-amino]-penta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of. [125I]-Bolton-Hunter Substance P to NK-1 receptors in the guinea pig cerebral... | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50283250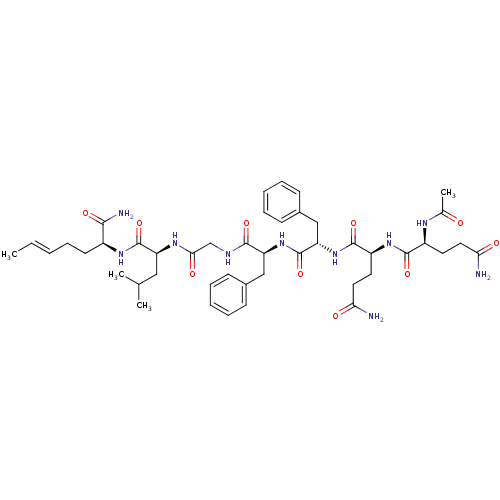 ((S)-2-[2-((S)-2-{(S)-2-[(S)-2-((S)-2-Acetylamino-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of. [125I]-Bolton-Hunter Substance P to NK-1 receptors in the guinea pig cerebral... | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50283257 ((S)-2-[2-((S)-2-{(S)-2-[(S)-2-((S)-2-Acetylamino-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 276 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of [3H]-Senktide to Tachykinin receptor 3 in the guinea pig cerebral cortex | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50283255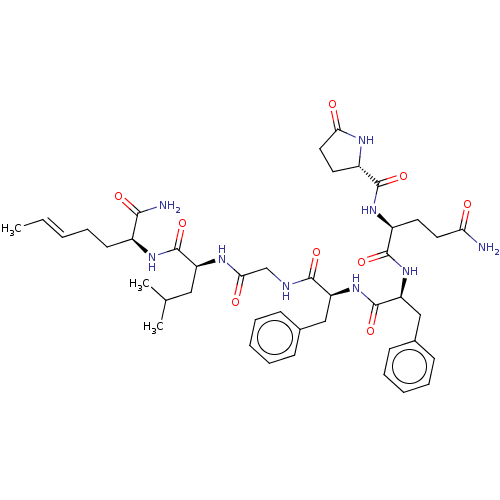 ((S)-2-[(5-Oxo-pyrrolidine-2-carbonyl)-amino]-penta...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 366 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of. [125I]-Bolton-Hunter Substance P to NK-1 receptors in the guinea pig cerebral... | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50283253 ((S)-2-[2-((S)-2-{(S)-2-[(S)-2-((S)-2-Acetylamino-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 533 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of [3H]-Senktide to Tachykinin receptor 3 in the guinea pig cerebral cortex | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (HAMSTER) | BDBM50283253 ((S)-2-[2-((S)-2-{(S)-2-[(S)-2-((S)-2-Acetylamino-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 637 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of. [125I]-Neurokinin A to NK-2 receptors in the hamster urinary bladder | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50283250 ((S)-2-[2-((S)-2-{(S)-2-[(S)-2-((S)-2-Acetylamino-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 882 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of [3H]-Senktide to Tachykinin receptor 3 in the guinea pig cerebral cortex | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50283252 ((S)-2-[2-((S)-2-{(S)-2-[(S)-2-((S)-2-Amino-4-carba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of [3H]-Senktide to Tachykinin receptor 3 in the guinea pig cerebral cortex | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (HAMSTER) | BDBM50283250 ((S)-2-[2-((S)-2-{(S)-2-[(S)-2-((S)-2-Acetylamino-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of. [125I]-Neurokinin A to NK-2 receptors in the hamster urinary bladder | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (HAMSTER) | BDBM50283257 ((S)-2-[2-((S)-2-{(S)-2-[(S)-2-((S)-2-Acetylamino-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of. [125I]-Neurokinin A to NK-2 receptors in the hamster urinary bladder | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50283255 ((S)-2-[(5-Oxo-pyrrolidine-2-carbonyl)-amino]-penta...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of [3H]-Senktide to Tachykinin receptor 3 in the guinea pig cerebral cortex | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (HAMSTER) | BDBM50283255 ((S)-2-[(5-Oxo-pyrrolidine-2-carbonyl)-amino]-penta...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of. [125I]-Neurokinin A to NK-2 receptors in the hamster urinary bladder | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (HAMSTER) | BDBM50283252 ((S)-2-[2-((S)-2-{(S)-2-[(S)-2-((S)-2-Amino-4-carba...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of. [125I]-Neurokinin A to NK-2 receptors in the hamster urinary bladder | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50450087 (CHEMBL2369769) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration producing half-maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancr... | Bioorg Med Chem Lett 4: 2263-2266 (1994) Article DOI: 10.1016/0960-894X(94)85022-4 BindingDB Entry DOI: 10.7270/Q2959HHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||