

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125628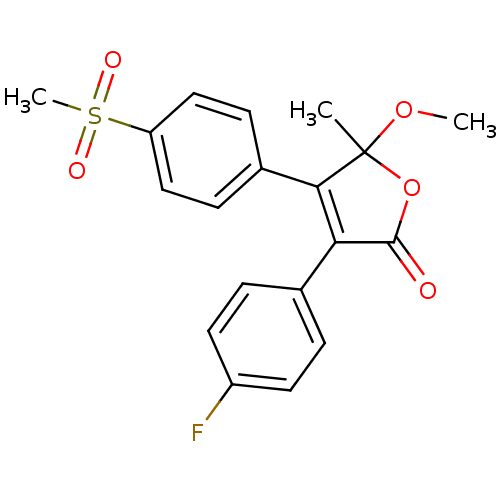 (3-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125613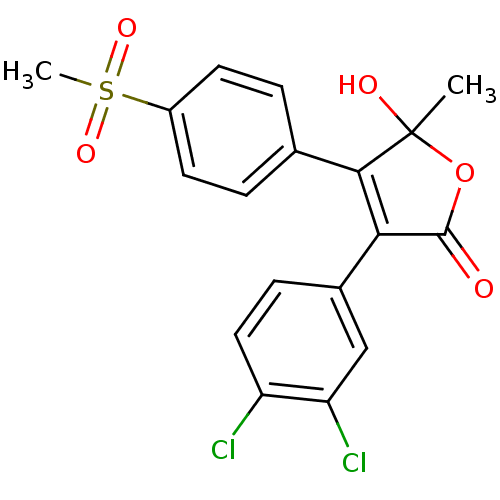 (3-(3,4-Dichloro-phenyl)-5-hydroxy-4-(4-methanesulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125628 (3-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Cyclooxygenase-2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125618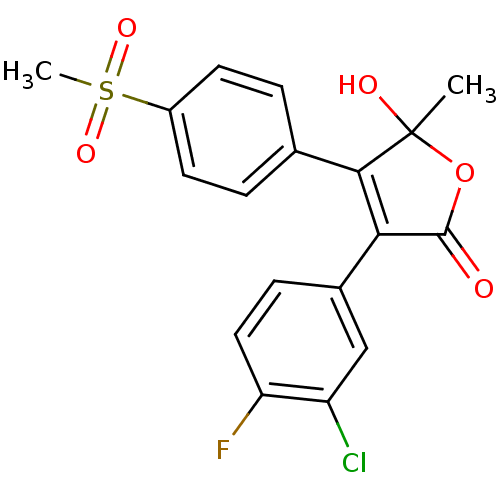 (3-(3-Chloro-4-fluoro-phenyl)-5-hydroxy-4-(4-methan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125624 (3-(4-Chloro-phenyl)-5-hydroxy-4-(4-methanesulfonyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125630 (5-Hydroxy-4-(4-methanesulfonyl-phenyl)-5-methyl-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 1 in the sensitive U937 microsome assay | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125631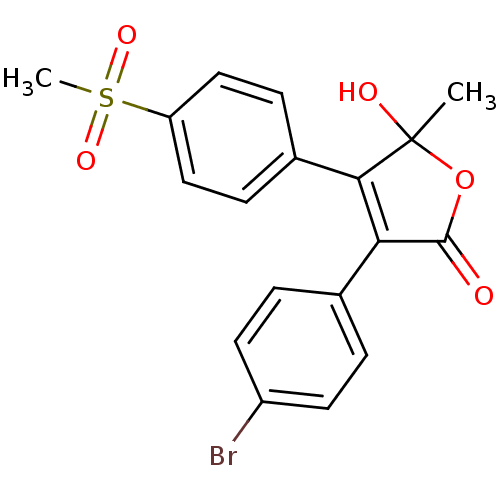 (3-(4-Bromo-phenyl)-5-hydroxy-4-(4-methanesulfonyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125626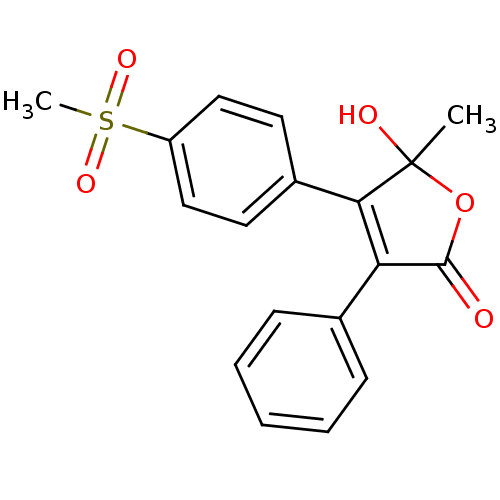 (5-Hydroxy-4-(4-methanesulfonyl-phenyl)-5-methyl-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125627 (3-(3,4-Difluoro-phenyl)-5-hydroxy-4-(4-methanesulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125622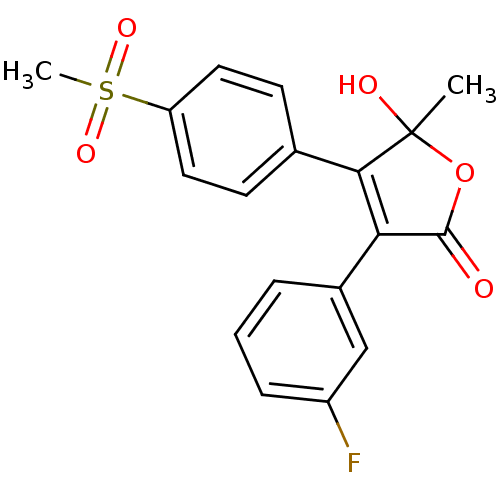 (3-(3-Fluoro-phenyl)-5-hydroxy-4-(4-methanesulfonyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125621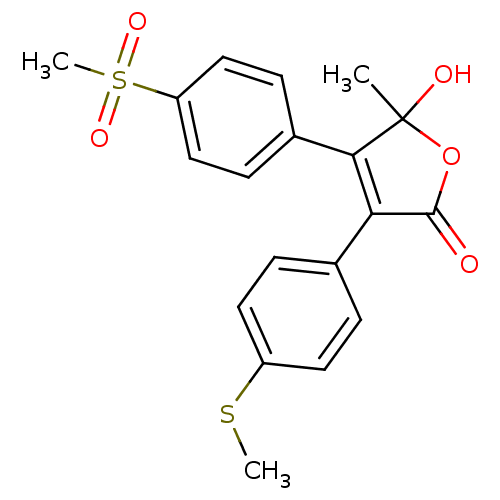 (5-Hydroxy-4-(4-methanesulfonyl-phenyl)-5-methyl-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125611 (3-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125612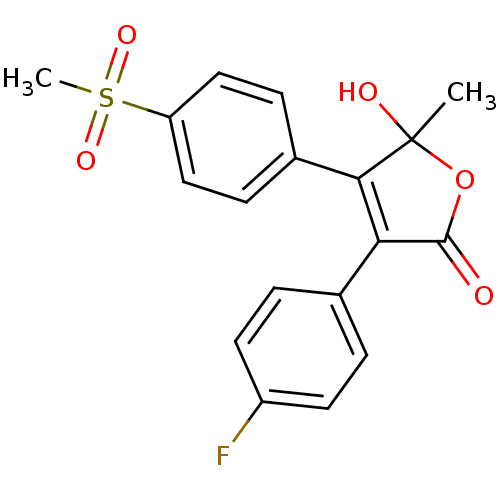 (3-(4-Fluoro-phenyl)-5-hydroxy-4-(4-methanesulfonyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125611 (3-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125615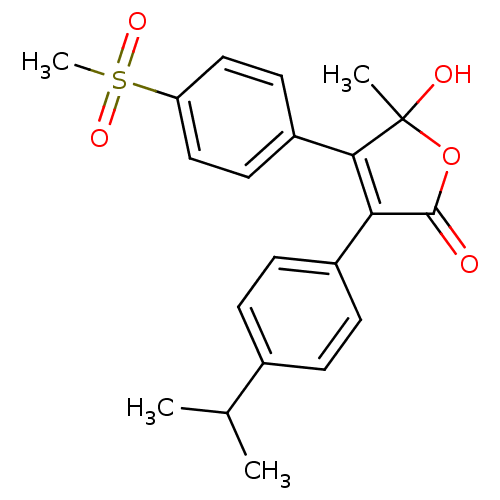 (5-Hydroxy-3-(4-isopropyl-phenyl)-4-(4-methanesulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125620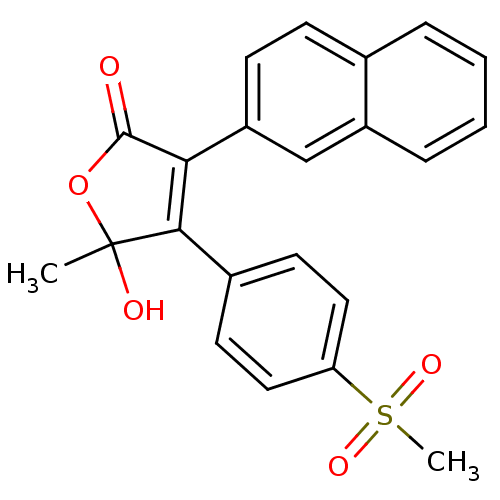 (5-Hydroxy-4-(4-methanesulfonyl-phenyl)-5-methyl-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125619 (5-Ethoxy-3-(4-fluoro-phenyl)-4-(4-methanesulfonyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125609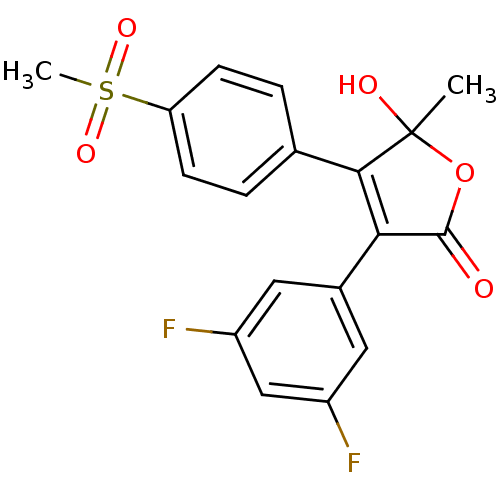 (3-(3,5-Difluoro-phenyl)-5-hydroxy-4-(4-methanesulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125623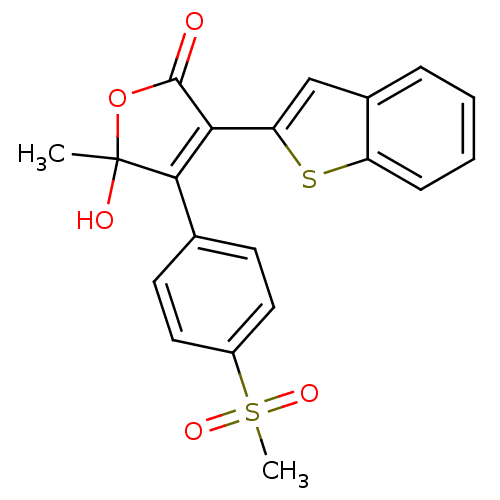 (3-Benzo[b]thiophen-2-yl-5-hydroxy-4-(4-methanesulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125610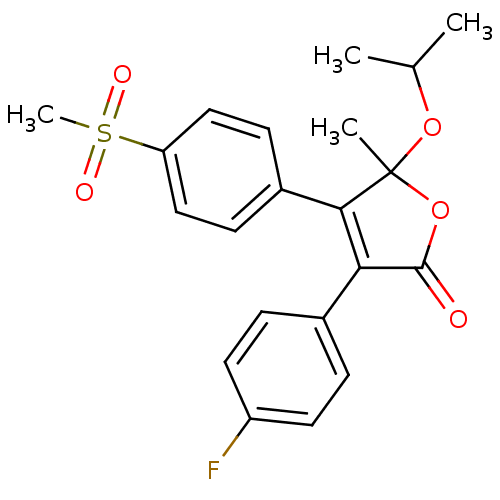 (3-(4-Fluoro-phenyl)-5-isopropoxy-4-(4-methanesulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125613 (3-(3,4-Dichloro-phenyl)-5-hydroxy-4-(4-methanesulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Cyclooxygenase-2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125617 (3-(2,4-Dichloro-phenyl)-5-hydroxy-4-(4-methanesulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125626 (5-Hydroxy-4-(4-methanesulfonyl-phenyl)-5-methyl-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 1 in the sensitive U937 microsome assay | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125631 (3-(4-Bromo-phenyl)-5-hydroxy-4-(4-methanesulfonyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125621 (5-Hydroxy-4-(4-methanesulfonyl-phenyl)-5-methyl-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125624 (3-(4-Chloro-phenyl)-5-hydroxy-4-(4-methanesulfonyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 1 in the sensitive U937 microsome assay | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125612 (3-(4-Fluoro-phenyl)-5-hydroxy-4-(4-methanesulfonyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Cyclooxygenase-2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125614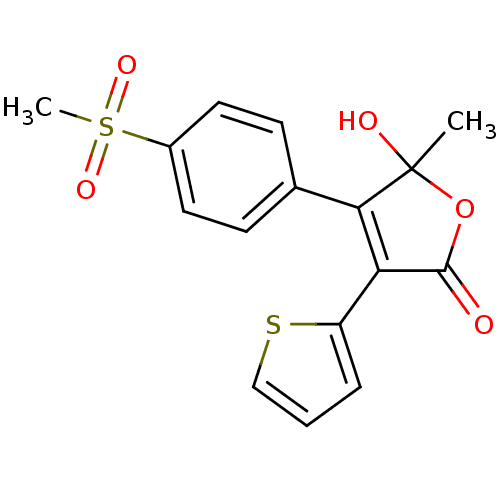 (5-Hydroxy-4-(4-methanesulfonyl-phenyl)-5-methyl-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125618 (3-(3-Chloro-4-fluoro-phenyl)-5-hydroxy-4-(4-methan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50125610 (3-(4-Fluoro-phenyl)-5-isopropoxy-4-(4-methanesulfo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 1 in the sensitive U937 microsome assay | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125620 (5-Hydroxy-4-(4-methanesulfonyl-phenyl)-5-methyl-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125623 (3-Benzo[b]thiophen-2-yl-5-hydroxy-4-(4-methanesulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory of human Prostaglandin G/H synthase 2 expressed in CHO cells. | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125615 (5-Hydroxy-3-(4-isopropyl-phenyl)-4-(4-methanesulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125625 (5-Hydroxy-3-(4-hydroxy-phenyl)-4-(4-methanesulfony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 1 in the sensitive U937 microsome assay | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125616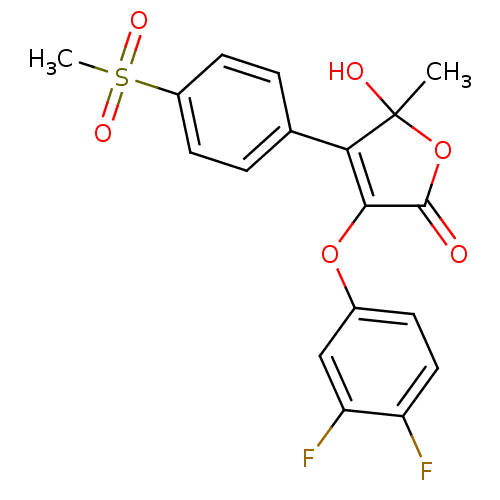 (3-(3,4-Difluoro-phenoxy)-5-hydroxy-4-(4-methanesul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 1 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125629 (5-Hydroxy-4-(4-methanesulfonyl-phenyl)-5-methyl-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125627 (3-(3,4-Difluoro-phenyl)-5-hydroxy-4-(4-methanesulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 1 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50125621 (5-Hydroxy-4-(4-methanesulfonyl-phenyl)-5-methyl-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 1 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125617 (3-(2,4-Dichloro-phenyl)-5-hydroxy-4-(4-methanesulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50125611 (3-(4-Fluoro-phenyl)-4-(4-methanesulfonyl-phenyl)-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 1 in the sensitive U937 microsome assay | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50125629 (5-Hydroxy-4-(4-methanesulfonyl-phenyl)-5-methyl-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 2 in human whole blood | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50125616 (3-(3,4-Difluoro-phenoxy)-5-hydroxy-4-(4-methanesul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 1 in the sensitive U937 microsome assay | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50125625 (5-Hydroxy-3-(4-hydroxy-phenyl)-4-(4-methanesulfony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against Prostaglandin G/H synthase 1 in the sensitive U937 microsome assay | Bioorg Med Chem Lett 13: 1195-8 (2003) BindingDB Entry DOI: 10.7270/Q2GT5MJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 82 total ) | Next | Last >> |