

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021119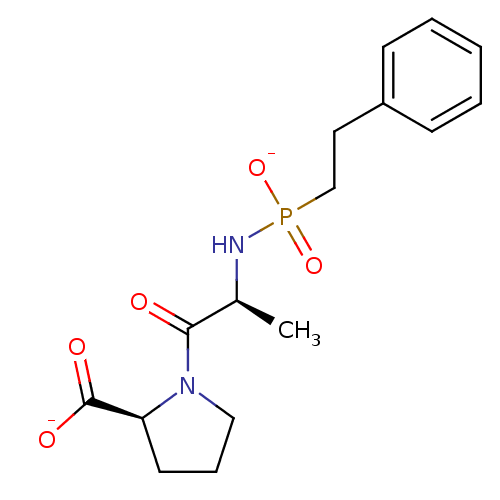 (CHEMBL34389 | disodium (2S)-1-((2S)-2-{[(2-phenyle...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021123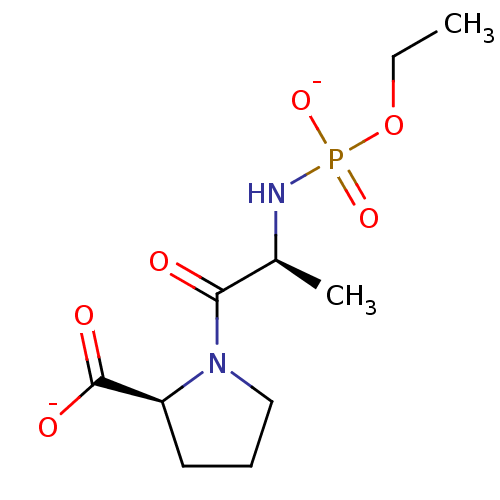 (CHEMBL30505 | dipotassium (2S)-1-{(2S)-2-[(ethoxyp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021118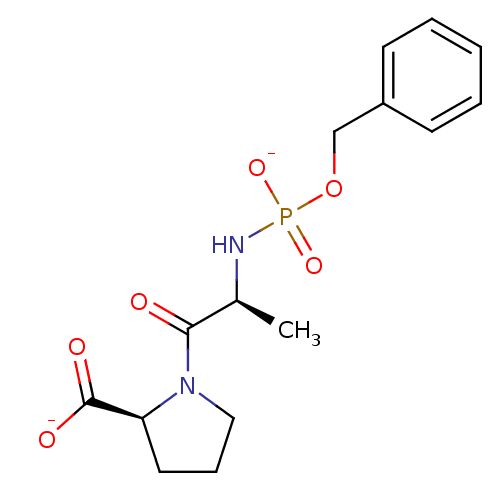 (CHEMBL30836 | dipotassium (2S)-1-((2S)-2-{[(benzyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021114 (CHEMBL282987 | tripotassium (2S)-1-[(2S)-2-(phosph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021121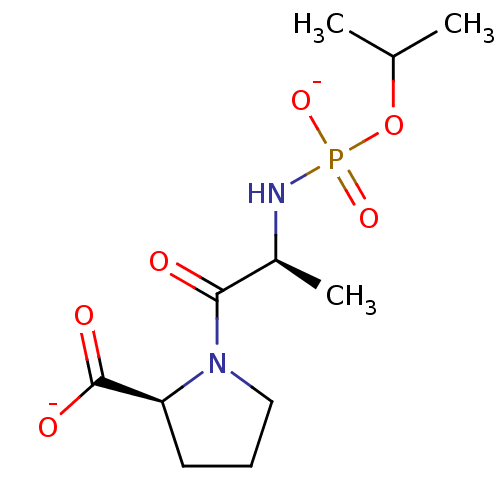 (CHEMBL286181 | dipotassium (2S)-1-{(2S)-2-[(isopro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021124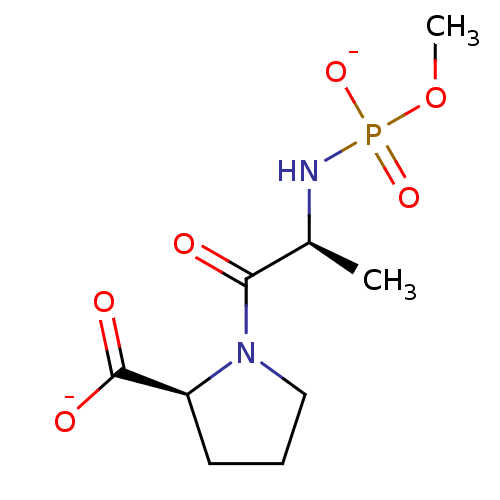 (CHEMBL34402 | dipotassium (2S)-1-{(2S)-2-[(methoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021116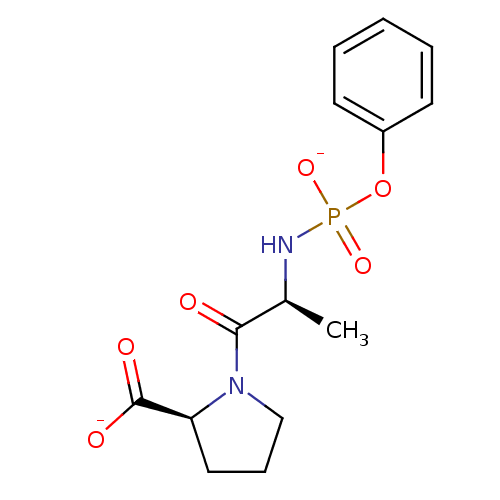 (CHEMBL33124 | dipotassium (2S)-1-{(2S)-2-[(phenoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021115 (CHEMBL33325 | disodium (2S)-1-((2S)-2-{[(4-nitroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant was evaluated against Angiotensin I converting enzyme from rabbit lungs | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021115 (CHEMBL33325 | disodium (2S)-1-((2S)-2-{[(4-nitroph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021122 (1-{2-[Bis-(4-nitro-phenoxy)-phosphorylamino]-propi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020390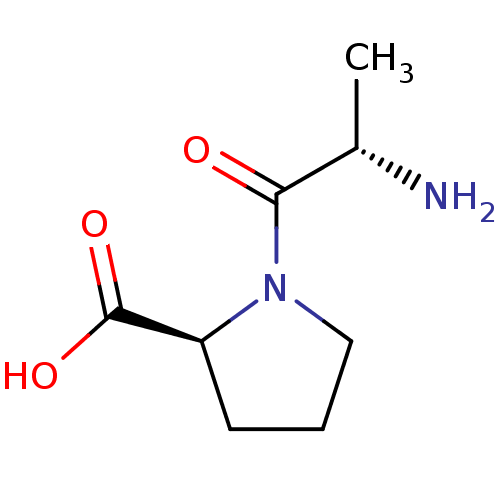 ((2S)-1-[(2S)-2-aminopropanoyl]pyrrolidine-2-carbox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.3 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant was evaluated against Angiotensin I converting enzyme from rabbit lungs at pH 8.3 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021120 (1-[2-(Diphenoxy-phosphorylamino)-propionyl]-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020390 ((2S)-1-[(2S)-2-aminopropanoyl]pyrrolidine-2-carbox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant was evaluated against Angiotensin I converting enzyme from rabbit lungs at pH 8 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021117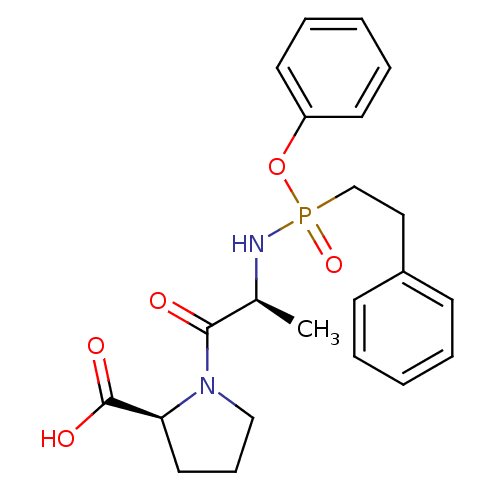 ((2S)-1-((2S)-2-{[phenylethyl(phenoxy)phosphoryl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021119 (CHEMBL34389 | disodium (2S)-1-((2S)-2-{[(2-phenyle...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme from rabbit lungs at pH 7.5 | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021114 (CHEMBL282987 | tripotassium (2S)-1-[(2S)-2-(phosph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme was evaluated | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021116 (CHEMBL33124 | dipotassium (2S)-1-{(2S)-2-[(phenoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | 8.0 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant was evaluated against Angiotensin I converting enzyme from rabbit lungs at pH 8 in NaCl buffer | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021116 (CHEMBL33124 | dipotassium (2S)-1-{(2S)-2-[(phenoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+7 | n/a | n/a | n/a | n/a | 8.0 | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Angiotensin I converting enzyme | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021116 (CHEMBL33124 | dipotassium (2S)-1-{(2S)-2-[(phenoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+8 | n/a | n/a | n/a | n/a | 8.0 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant was evaluated against Angiotensin I converting enzyme from rabbit lungs at pH 8 in TRIS phosphate/borate buffer | J Med Chem 28: 1422-7 (1985) BindingDB Entry DOI: 10.7270/Q2Z60N24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||