

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin D (Homo sapiens (Human)) | BDBM50010851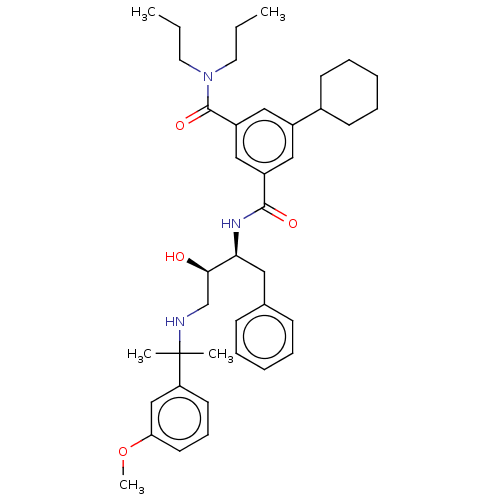 (CHEMBL3264810) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010852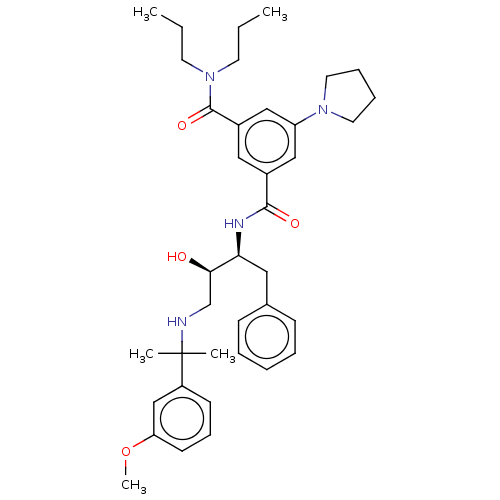 (CHEMBL3264811) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010851 (CHEMBL3264810) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010849 (CHEMBL3264808) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010894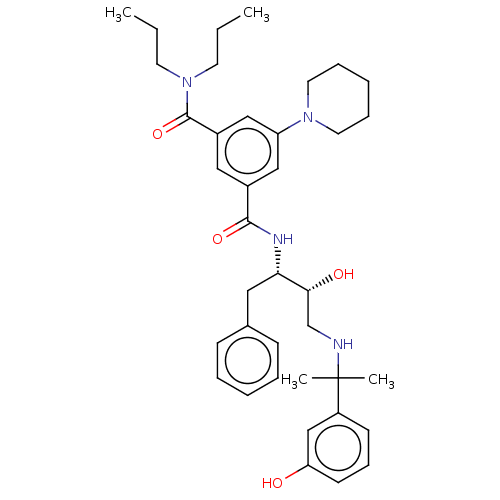 (CHEMBL3264800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010882 (CHEMBL3264799) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010854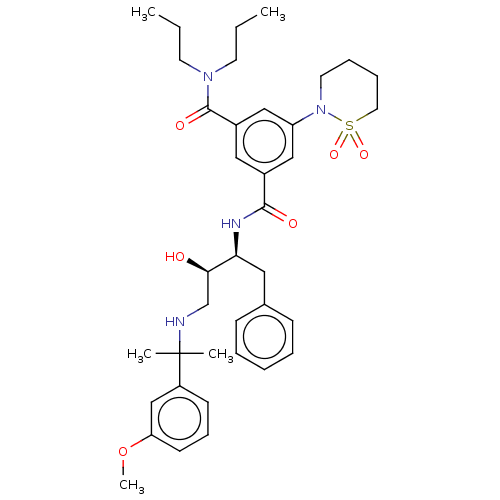 (CHEMBL587764 | TCMDC-134674) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010896 (CHEMBL3264801) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010853 (CHEMBL3264812) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010843 (CHEMBL3264805) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010882 (CHEMBL3264799) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010853 (CHEMBL3264812) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010894 (CHEMBL3264800) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010846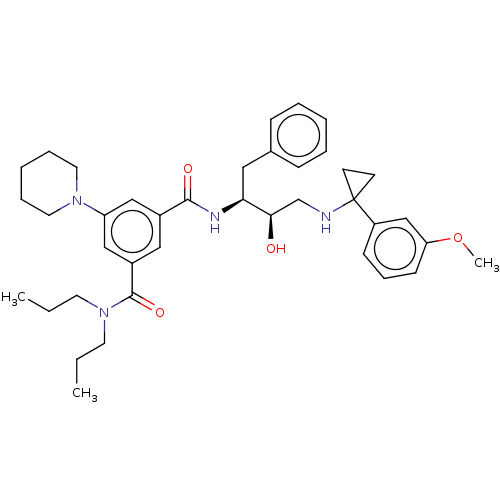 (CHEMBL3264806) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010896 (CHEMBL3264801) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010848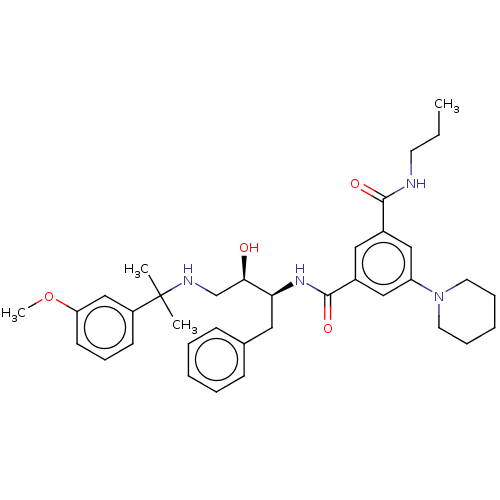 (CHEMBL3259870) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010852 (CHEMBL3264811) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010856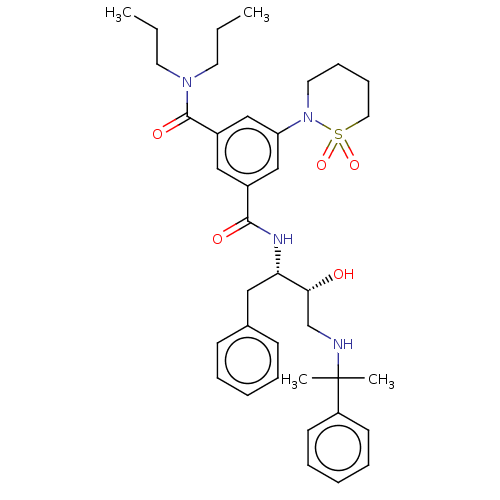 (CHEMBL3264797) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010899 (CHEMBL3264803) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010854 (CHEMBL587764 | TCMDC-134674) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010846 (CHEMBL3264806) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010839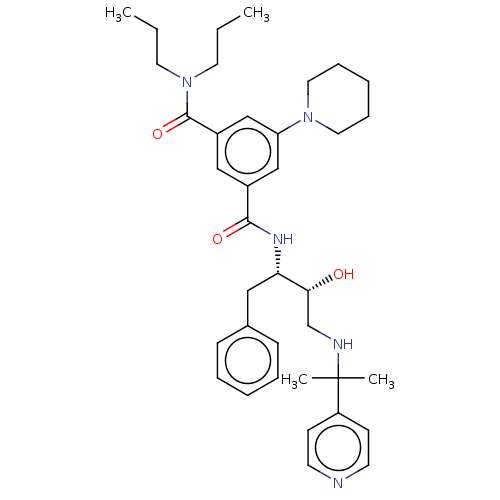 (CHEMBL3264804) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010899 (CHEMBL3264803) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010843 (CHEMBL3264805) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010847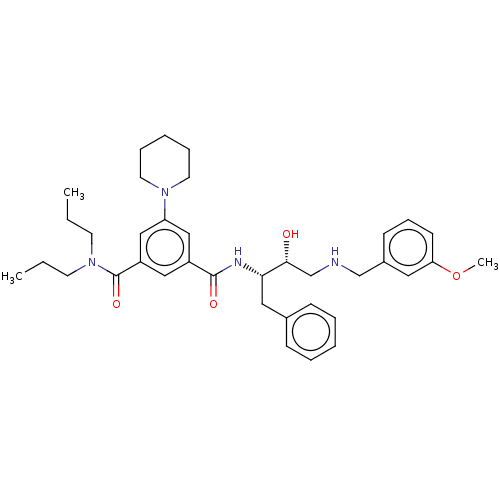 (CHEMBL3264807) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010851 (CHEMBL3264810) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010853 (CHEMBL3264812) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010839 (CHEMBL3264804) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010882 (CHEMBL3264799) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010856 (CHEMBL3264797) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010846 (CHEMBL3264806) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010897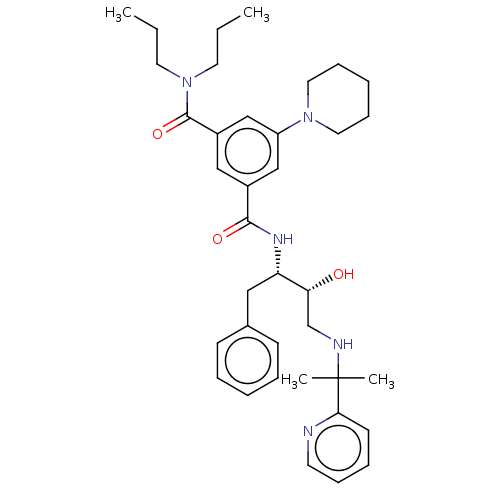 (CHEMBL3264802) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010899 (CHEMBL3264803) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010894 (CHEMBL3264800) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010845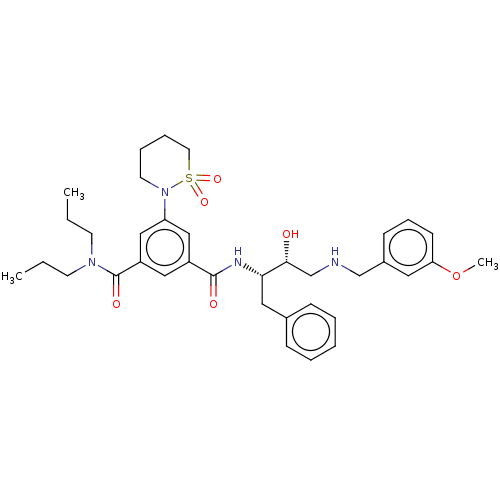 (CHEMBL581498 | TCMDC-138893) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010896 (CHEMBL3264801) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010852 (CHEMBL3264811) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010839 (CHEMBL3264804) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010848 (CHEMBL3259870) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50010850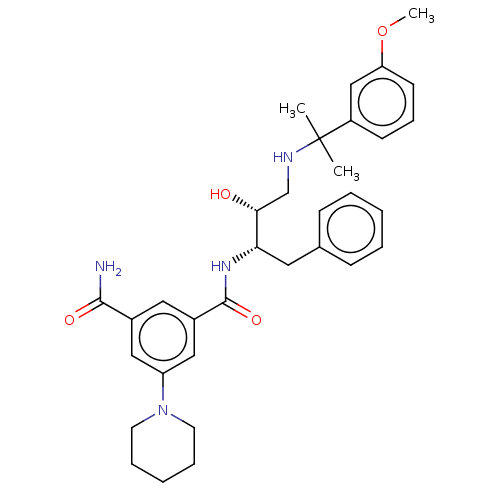 (CHEMBL3264809) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of human cathepsin-D using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET assay | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010849 (CHEMBL3264808) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010843 (CHEMBL3264805) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010847 (CHEMBL3264807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010854 (CHEMBL587764 | TCMDC-134674) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010848 (CHEMBL3259870) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010897 (CHEMBL3264802) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010849 (CHEMBL3264808) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010856 (CHEMBL3264797) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50010845 (CHEMBL581498 | TCMDC-138893) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-2 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50010897 (CHEMBL3264802) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Latvian Institute of Organic Synthesis Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin-1 using DABCYL-Glu-Arg-Nle-Phe-Leu-Ser-Phe-Pro-EDANS as substrate preincubated for 30 mins by FRET ass... | ACS Med Chem Lett 5: 373-7 (2014) Article DOI: 10.1021/ml4004952 BindingDB Entry DOI: 10.7270/Q26W9CMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 60 total ) | Next | Last >> |