

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antiviral activity as inhibition of human liver microsomal Cytochrome P450 3A4 | Bioorg Med Chem Lett 8: 3531-6 (1999) BindingDB Entry DOI: 10.7270/Q2NC60CH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50073216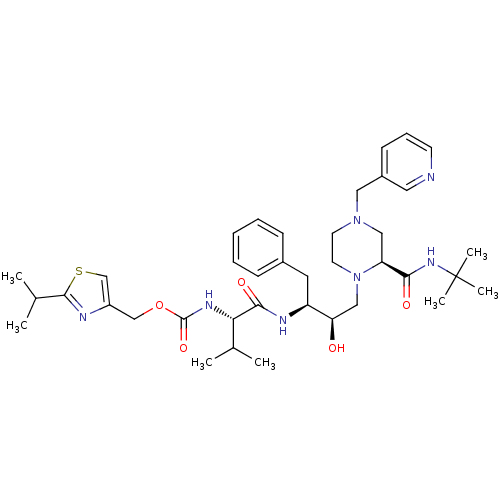 (CHEMBL324033 | {(S)-1-[(1S,2R)-1-Benzyl-3-((S)-2-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antiviral activity as inhibition of human liver microsomal Cytochrome P450 3A4 | Bioorg Med Chem Lett 8: 3531-6 (1999) BindingDB Entry DOI: 10.7270/Q2NC60CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50073219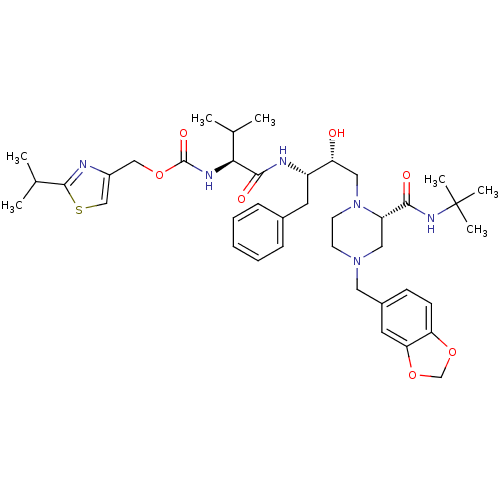 (CHEMBL116772 | {(S)-1-[(1S,2R)-3-((S)-4-Benzo[1,3]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antiviral activity as inhibition of human liver microsomal Cytochrome P450 3A4 | Bioorg Med Chem Lett 8: 3531-6 (1999) BindingDB Entry DOI: 10.7270/Q2NC60CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50215312 (CHEMBL3706711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antiviral activity as inhibition of human liver microsomal Cytochrome P450 3A4 | Bioorg Med Chem Lett 8: 3531-6 (1999) BindingDB Entry DOI: 10.7270/Q2NC60CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50073235 (CHEMBL263179 | {(S)-1-[(1S,2R)-1-Benzyl-3-((S)-2-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antiviral activity as inhibition of human liver microsomal Cytochrome P450 3A4 | Bioorg Med Chem Lett 8: 3531-6 (1999) BindingDB Entry DOI: 10.7270/Q2NC60CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50073218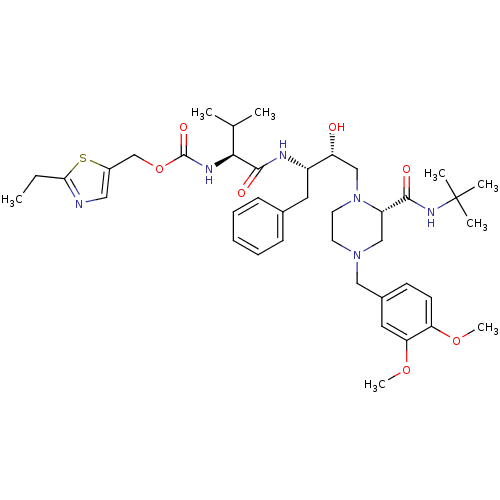 (((S)-1-{(1S,2R)-1-Benzyl-3-[(S)-2-tert-butylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antiviral activity as inhibition of human liver microsomal Cytochrome P450 3A4 | Bioorg Med Chem Lett 8: 3531-6 (1999) BindingDB Entry DOI: 10.7270/Q2NC60CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50073225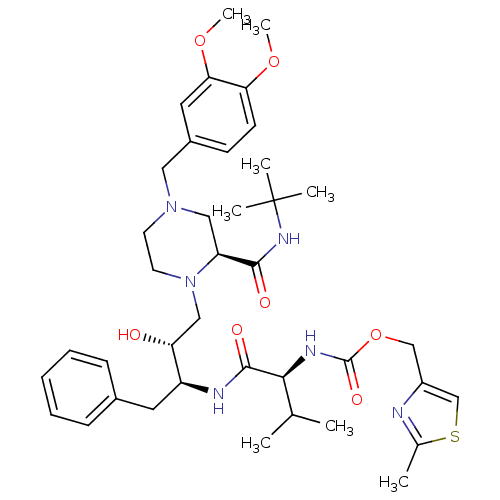 (((S)-1-{(1S,2R)-1-Benzyl-3-[(S)-2-tert-butylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antiviral activity as inhibition of human liver microsomal Cytochrome P450 3A4 | Bioorg Med Chem Lett 8: 3531-6 (1999) BindingDB Entry DOI: 10.7270/Q2NC60CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||