

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50080528 (3-((R)-2-((1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Inhibition constant(Ki) for inhibition of PPIase activity of human FK506 binding protein 12 (Conc=14 nM) of FKBPsfamily | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 (Homo sapiens (Human)) | BDBM50080528 (3-((R)-2-((1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Inhibition constant(Ki) for inhibition of PPIase activity of rabbit FK506 binding protein 52 (Conc=52 nM) of FKBPsfamily | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP14 (Homo sapiens (Human)) | BDBM50080528 (3-((R)-2-((1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Inhibition constant(Ki) for inhibition of PPIase activity of Photobacterium sp. FK506 binding protein 22 (Conc=41 nM) of FKBPsfamily | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP3 (Homo sapiens (Human)) | BDBM50080528 (3-((R)-2-((1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl)...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Inhibition constant(Ki) for inhibition of PPIase activity of L. pneumophilia FK506 binding protein 25 (Conc=40 nM) of FKBPsfamily | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 4 (Homo sapiens (Human)) | BDBM50080528 (3-((R)-2-((1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Inhibition constant(Ki) for inhibition of PPIase activity of E. coli parvulin (Conc=4 nM) of Parvulins sfamily | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50080528 (3-((R)-2-((1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Inhibition constant(Ki) for inhibition of PPIase activity of human Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Conc=4 nM) of Parvulins fa... | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50080528 (3-((R)-2-((1S,3S,5S)-3,5-dimethyl-2-oxocyclohexyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory effect on human FK506 binding protein 12 by means of protease-coupled PPIase assay | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50080534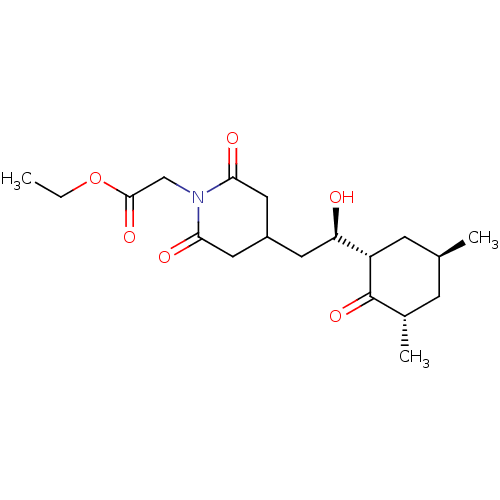 (CHEMBL333448 | ethyl (4-{(2R)-2-[(1S,3S,5S)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory effect on human FK506 binding protein 12 by means of protease-coupled PPIase assay | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50080531 (2-{4-[(R)-2-((1S,3S,5S)-3,5-Dimethyl-2-oxo-cyclohe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory effect on human FK506 binding protein 12 by means of protease-coupled PPIase assay | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50080530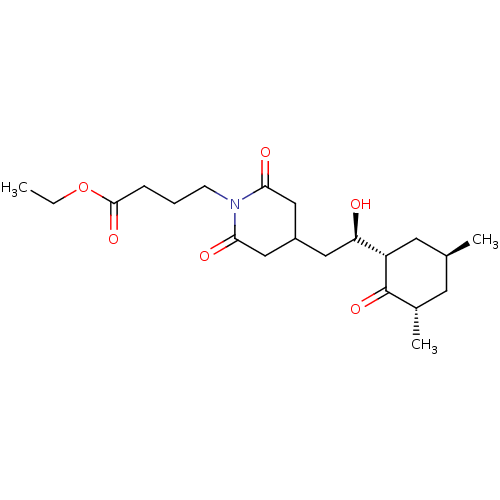 (4-{4-[(R)-2-((1S,3S,5S)-3,5-Dimethyl-2-oxo-cyclohe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory effect on human FK506 binding protein 12 by means of protease-coupled PPIase assay | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50080532 (4-{4-[(R)-2-((1S,3S,5S)-3,5-Dimethyl-2-oxo-cyclohe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory effect on human FK506 binding protein 12 by means of protease-coupled PPIase assay | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50080535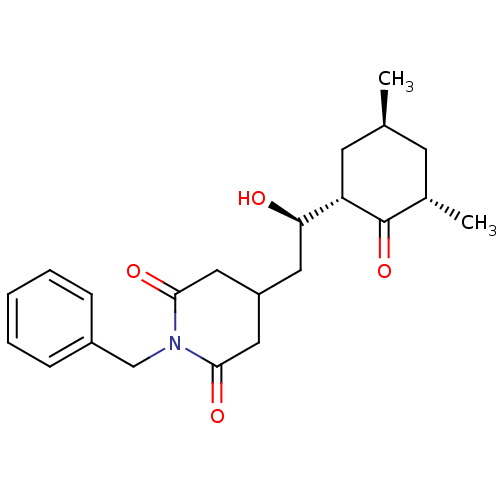 (1-Benzyl-4-[(R)-2-((1S,3S,5S)-3,5-dimethyl-2-oxo-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory effect on human FK506 binding protein 12 by means of protease-coupled PPIase assay | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50080527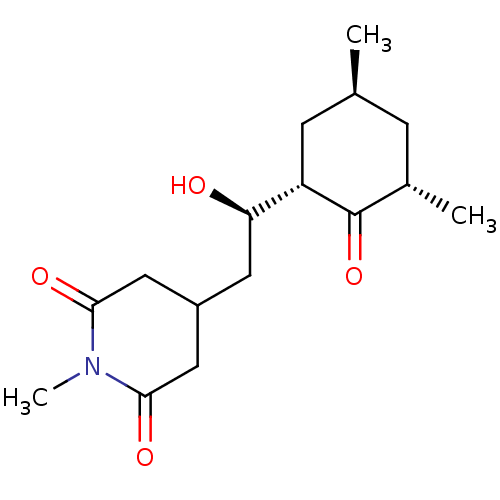 (4-[(R)-2-((1S,3S,5S)-3,5-Dimethyl-2-oxo-cyclohexyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory effect on human FK506 binding protein 12 by means of protease-coupled PPIase assay | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50080536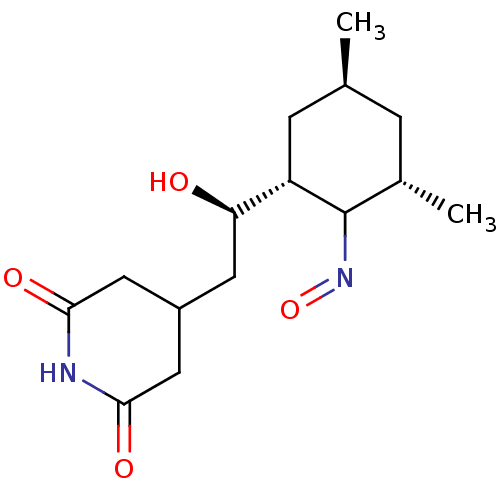 (4-((R)-2-Hydroxy-2-{(1R,3S,5S)-2-[(Z)-hydroxyimino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory effect on human FK506 binding protein 12 by means of protease-coupled PPIase assay | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50080533 (CHEMBL331865 | {4-[(R)-2-(Adamantan-1-ylcarbamoylo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory effect on human FK506 binding protein 12 by means of protease-coupled PPIase assay | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50080529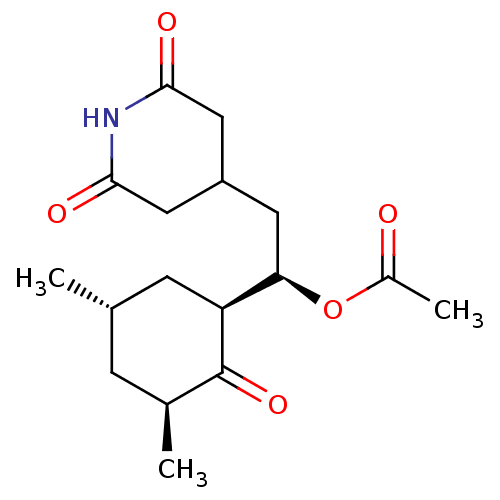 (Acetic acid (R)-1-((1S,3S,5S)-3,5-dimethyl-2-oxo-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck Research Unit Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory effect on human FK506 binding protein 12 by means of protease-coupled PPIase assay | J Med Chem 42: 3615-22 (1999) Article DOI: 10.1021/jm991038t BindingDB Entry DOI: 10.7270/Q2DB8124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||