 Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50009761
Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50009761 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50065115

(3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C27H21ClFN3O2/c1-17-8-9-19(29)13-23(17)26(33)30-20-10-11-22(24(28)14-20)27(34)32-16-21-6-4-12-31(21)15-18-5-2-3-7-25(18)32/h2-14H,15-16H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087550
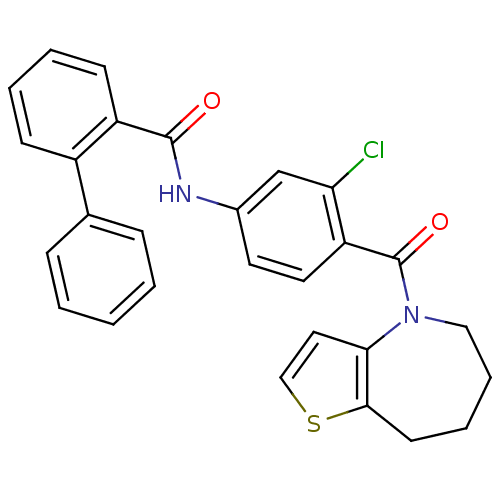
(Biphenyl-2-carboxylic acid [3-chloro-4-(5,6,7,8-te...)Show SMILES Clc1cc(NC(=O)c2ccccc2-c2ccccc2)ccc1C(=O)N1CCCCc2sccc12 Show InChI InChI=1S/C28H23ClN2O2S/c29-24-18-20(30-27(32)22-11-5-4-10-21(22)19-8-2-1-3-9-19)13-14-23(24)28(33)31-16-7-6-12-26-25(31)15-17-34-26/h1-5,8-11,13-15,17-18H,6-7,12,16H2,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087541

(Biphenyl-2-carboxylic acid [4-(5H,11H-benzo[e]pyrr...)Show SMILES Clc1cc(NC(=O)c2ccccc2-c2ccccc2)ccc1C(=O)N1Cc2cccn2Cc2ccccc12 Show InChI InChI=1S/C32H24ClN3O2/c33-29-19-24(34-31(37)27-14-6-5-13-26(27)22-9-2-1-3-10-22)16-17-28(29)32(38)36-21-25-12-8-18-35(25)20-23-11-4-7-15-30(23)36/h1-19H,20-21H2,(H,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50087551
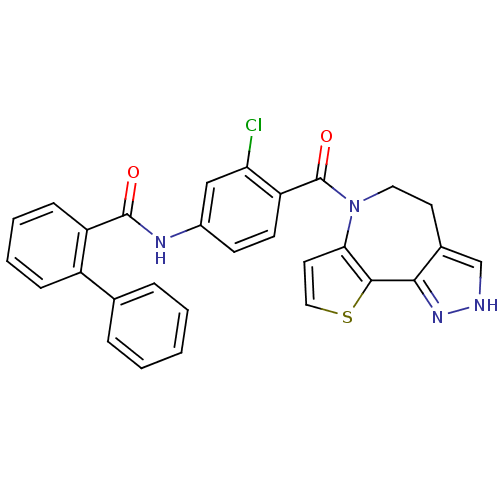
(Biphenyl-2-carboxylic acid [3-chloro-4-(4,5-dihydr...)Show SMILES Clc1cc(NC(=O)c2ccccc2-c2ccccc2)ccc1C(=O)N1CCc2c[nH]nc2-c2sccc12 Show InChI InChI=1S/C29H21ClN4O2S/c30-24-16-20(32-28(35)22-9-5-4-8-21(22)18-6-2-1-3-7-18)10-11-23(24)29(36)34-14-12-19-17-31-33-26(19)27-25(34)13-15-37-27/h1-11,13,15-17H,12,14H2,(H,31,33)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50065115

(3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C27H21ClFN3O2/c1-17-8-9-19(29)13-23(17)26(33)30-20-10-11-22(24(28)14-20)27(34)32-16-21-6-4-12-31(21)15-18-5-2-3-7-25(18)32/h2-14H,15-16H2,1H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087552

(Biphenyl-2-carboxylic acid [4-(5H,11H-benzo[e]pyrr...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1Cc2cccn2Cc2ccccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C32H25N3O2/c36-31(29-14-6-5-13-28(29)23-9-2-1-3-10-23)33-26-18-16-24(17-19-26)32(37)35-22-27-12-8-20-34(27)21-25-11-4-7-15-30(25)35/h1-20H,21-22H2,(H,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50087541

(Biphenyl-2-carboxylic acid [4-(5H,11H-benzo[e]pyrr...)Show SMILES Clc1cc(NC(=O)c2ccccc2-c2ccccc2)ccc1C(=O)N1Cc2cccn2Cc2ccccc12 Show InChI InChI=1S/C32H24ClN3O2/c33-29-19-24(34-31(37)27-14-6-5-13-26(27)22-9-2-1-3-10-22)16-17-28(29)32(38)36-21-25-12-8-18-35(25)20-23-11-4-7-15-30(23)36/h1-19H,20-21H2,(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50065110

(CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...)Show SMILES Clc1ccc(C(=O)Nc2ccc(cc2)C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2N3O2/c27-19-9-12-22(23(28)14-19)25(32)29-20-10-7-17(8-11-20)26(33)31-16-21-5-3-13-30(21)15-18-4-1-2-6-24(18)31/h1-14H,15-16H2,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50065110

(CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...)Show SMILES Clc1ccc(C(=O)Nc2ccc(cc2)C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2N3O2/c27-19-9-12-22(23(28)14-19)25(32)29-20-10-7-17(8-11-20)26(33)31-16-21-5-3-13-30(21)15-18-4-1-2-6-24(18)31/h1-14H,15-16H2,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087543
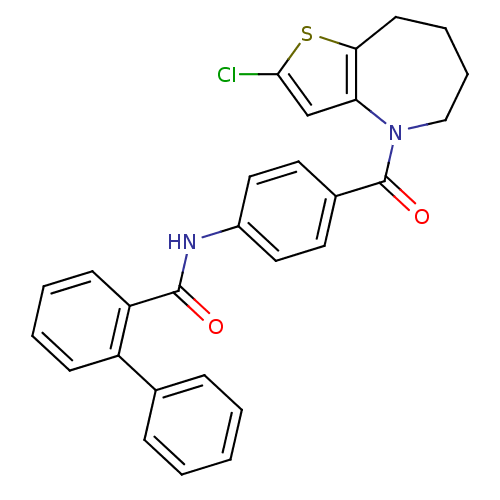
(Biphenyl-2-carboxylic acid [4-(2-chloro-5,6,7,8-te...)Show SMILES Clc1cc2N(CCCCc2s1)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C28H23ClN2O2S/c29-26-18-24-25(34-26)12-6-7-17-31(24)28(33)20-13-15-21(16-14-20)30-27(32)23-11-5-4-10-22(23)19-8-2-1-3-9-19/h1-5,8-11,13-16,18H,6-7,12,17H2,(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50087552

(Biphenyl-2-carboxylic acid [4-(5H,11H-benzo[e]pyrr...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1Cc2cccn2Cc2ccccc12)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C32H25N3O2/c36-31(29-14-6-5-13-28(29)23-9-2-1-3-10-23)33-26-18-16-24(17-19-26)32(37)35-22-27-12-8-20-34(27)21-25-11-4-7-15-30(25)35/h1-20H,21-22H2,(H,33,36) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity for rat V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50087542
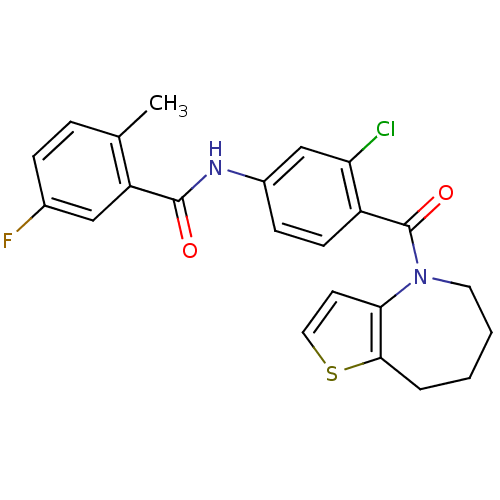
(CHEMBL350089 | N-[3-Chloro-4-(5,6,7,8-tetrahydro-t...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(C(=O)N2CCCCc3sccc23)c(Cl)c1 Show InChI InChI=1S/C23H20ClFN2O2S/c1-14-5-6-15(25)12-18(14)22(28)26-16-7-8-17(19(24)13-16)23(29)27-10-3-2-4-21-20(27)9-11-30-21/h5-9,11-13H,2-4,10H2,1H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087554
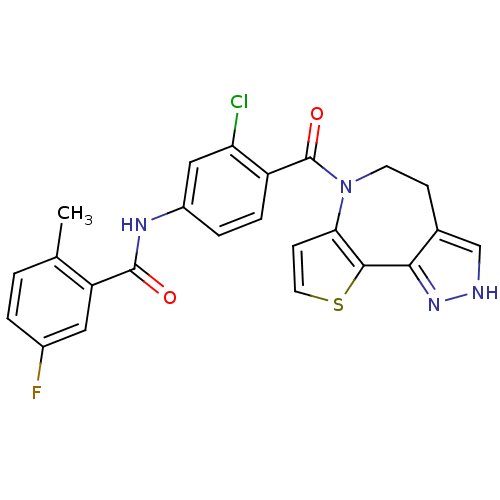
(CHEMBL162748 | N-[3-Chloro-4-(4,5-dihydro-2H-9-thi...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(C(=O)N2CCc3c[nH]nc3-c3sccc23)c(Cl)c1 Show InChI InChI=1S/C24H18ClFN4O2S/c1-13-2-3-15(26)10-18(13)23(31)28-16-4-5-17(19(25)11-16)24(32)30-8-6-14-12-27-29-21(14)22-20(30)7-9-33-22/h2-5,7,9-12H,6,8H2,1H3,(H,27,29)(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro for its binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50065110

(CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...)Show SMILES Clc1ccc(C(=O)Nc2ccc(cc2)C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2N3O2/c27-19-9-12-22(23(28)14-19)25(32)29-20-10-7-17(8-11-20)26(33)31-16-21-5-3-13-30(21)15-18-4-1-2-6-24(18)31/h1-14H,15-16H2,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50087541

(Biphenyl-2-carboxylic acid [4-(5H,11H-benzo[e]pyrr...)Show SMILES Clc1cc(NC(=O)c2ccccc2-c2ccccc2)ccc1C(=O)N1Cc2cccn2Cc2ccccc12 Show InChI InChI=1S/C32H24ClN3O2/c33-29-19-24(34-31(37)27-14-6-5-13-26(27)22-9-2-1-3-10-22)16-17-28(29)32(38)36-21-25-12-8-18-35(25)20-23-11-4-7-15-30(23)36/h1-19H,20-21H2,(H,34,37) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity for rat V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087549

(2,4-Dichloro-N-[4-(2-chloro-5,6,7,8-tetrahydro-thi...)Show SMILES Clc1cc2N(CCCCc2s1)C(=O)c1ccc(NC(=O)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C22H17Cl3N2O2S/c23-14-6-9-16(17(24)11-14)21(28)26-15-7-4-13(5-8-15)22(29)27-10-2-1-3-19-18(27)12-20(25)30-19/h4-9,11-12H,1-3,10H2,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50065110

(CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...)Show SMILES Clc1ccc(C(=O)Nc2ccc(cc2)C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C26H19Cl2N3O2/c27-19-9-12-22(23(28)14-19)25(32)29-20-10-7-17(8-11-20)26(33)31-16-21-5-3-13-30(21)15-18-4-1-2-6-24(18)31/h1-14H,15-16H2,(H,29,32) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity for rat V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087547
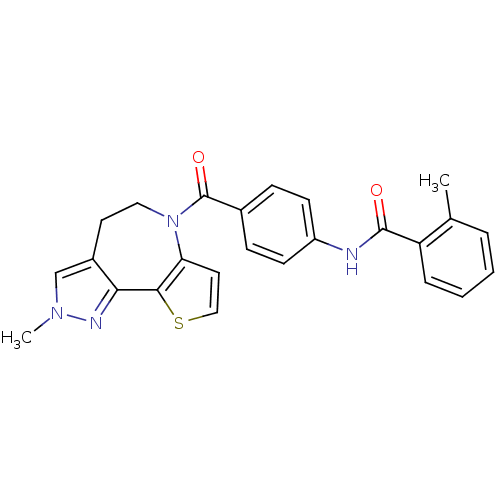
(2-Methyl-N-[4-(2-methyl-4,5-dihydro-2H-9-thia-1,2,...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCc2cn(C)nc2-c2sccc12 Show InChI InChI=1S/C25H22N4O2S/c1-16-5-3-4-6-20(16)24(30)26-19-9-7-17(8-10-19)25(31)29-13-11-18-15-28(2)27-22(18)23-21(29)12-14-32-23/h3-10,12,14-15H,11,13H2,1-2H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087553
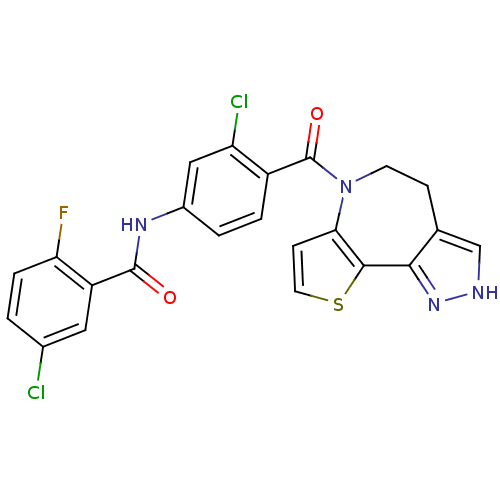
(CHEMBL351970 | N-[3-Chloro-4-(4,5-dihydro-2H-9-thi...)Show SMILES Fc1ccc(Cl)cc1C(=O)Nc1ccc(C(=O)N2CCc3c[nH]nc3-c3sccc23)c(Cl)c1 Show InChI InChI=1S/C23H15Cl2FN4O2S/c24-13-1-4-18(26)16(9-13)22(31)28-14-2-3-15(17(25)10-14)23(32)30-7-5-12-11-27-29-20(12)21-19(30)6-8-33-21/h1-4,6,8-11H,5,7H2,(H,27,29)(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro for its binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087545
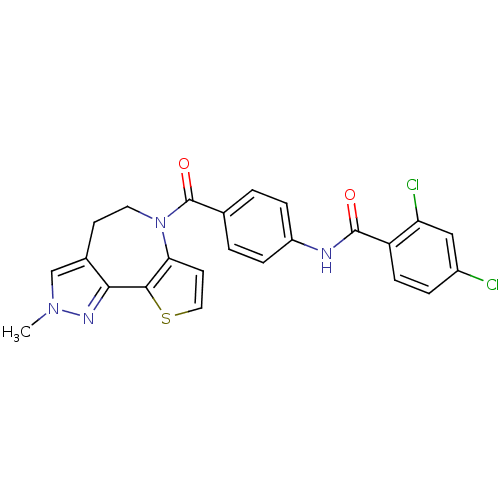
(2,4-Dichloro-N-[4-(2-methyl-4,5-dihydro-2H-9-thia-...)Show SMILES Cn1cc2CCN(C(=O)c3ccc(NC(=O)c4ccc(Cl)cc4Cl)cc3)c3ccsc3-c2n1 Show InChI InChI=1S/C24H18Cl2N4O2S/c1-29-13-15-8-10-30(20-9-11-33-22(20)21(15)28-29)24(32)14-2-5-17(6-3-14)27-23(31)18-7-4-16(25)12-19(18)26/h2-7,9,11-13H,8,10H2,1H3,(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087555
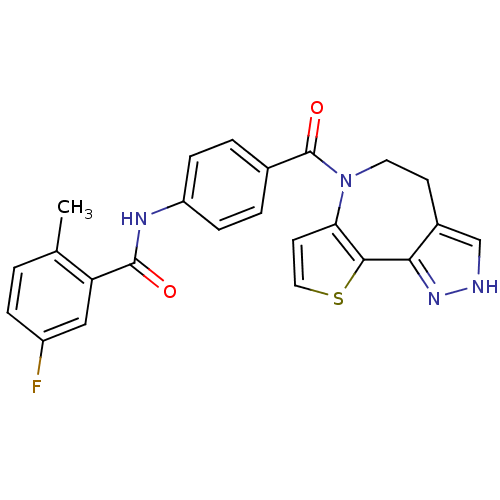
(CHEMBL350090 | N-[4-(4,5-Dihydro-2H-9-thia-1,2,6-t...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(cc1)C(=O)N1CCc2c[nH]nc2-c2sccc12 Show InChI InChI=1S/C24H19FN4O2S/c1-14-2-5-17(25)12-19(14)23(30)27-18-6-3-15(4-7-18)24(31)29-10-8-16-13-26-28-21(16)22-20(29)9-11-32-22/h2-7,9,11-13H,8,10H2,1H3,(H,26,28)(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro for its binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50087555

(CHEMBL350090 | N-[4-(4,5-Dihydro-2H-9-thia-1,2,6-t...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(cc1)C(=O)N1CCc2c[nH]nc2-c2sccc12 Show InChI InChI=1S/C24H19FN4O2S/c1-14-2-5-17(25)12-19(14)23(30)27-18-6-3-15(4-7-18)24(31)29-10-8-16-13-26-28-21(16)22-20(29)9-11-32-22/h2-7,9,11-13H,8,10H2,1H3,(H,26,28)(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50087543

(Biphenyl-2-carboxylic acid [4-(2-chloro-5,6,7,8-te...)Show SMILES Clc1cc2N(CCCCc2s1)C(=O)c1ccc(NC(=O)c2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C28H23ClN2O2S/c29-26-18-24-25(34-26)12-6-7-17-31(24)28(33)20-13-15-21(16-14-20)30-27(32)23-11-5-4-10-22(23)19-8-2-1-3-9-19/h1-5,8-11,13-16,18H,6-7,12,17H2,(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50087550

(Biphenyl-2-carboxylic acid [3-chloro-4-(5,6,7,8-te...)Show SMILES Clc1cc(NC(=O)c2ccccc2-c2ccccc2)ccc1C(=O)N1CCCCc2sccc12 Show InChI InChI=1S/C28H23ClN2O2S/c29-24-18-20(30-27(32)22-11-5-4-10-21(22)19-8-2-1-3-9-19)13-14-23(24)28(33)31-16-7-6-12-26-25(31)15-17-34-26/h1-5,8-11,13-15,17-18H,6-7,12,16H2,(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50087542

(CHEMBL350089 | N-[3-Chloro-4-(5,6,7,8-tetrahydro-t...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(C(=O)N2CCCCc3sccc23)c(Cl)c1 Show InChI InChI=1S/C23H20ClFN2O2S/c1-14-5-6-15(25)12-18(14)22(28)26-16-7-8-17(19(24)13-16)23(29)27-10-3-2-4-21-20(27)9-11-30-21/h5-9,11-13H,2-4,10H2,1H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087548

(CHEMBL350383 | N-[4-(4,5-Dihydro-2H-9-thia-1,2,6-t...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCc2c[nH]nc2-c2sccc12 Show InChI InChI=1S/C24H20N4O2S/c1-15-4-2-3-5-19(15)23(29)26-18-8-6-16(7-9-18)24(30)28-12-10-17-14-25-27-21(17)22-20(28)11-13-31-22/h2-9,11,13-14H,10,12H2,1H3,(H,25,27)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro for its binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50087548

(CHEMBL350383 | N-[4-(4,5-Dihydro-2H-9-thia-1,2,6-t...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCc2c[nH]nc2-c2sccc12 Show InChI InChI=1S/C24H20N4O2S/c1-15-4-2-3-5-19(15)23(29)26-18-8-6-16(7-9-18)24(30)28-12-10-17-14-25-27-21(17)22-20(28)11-13-31-22/h2-9,11,13-14H,10,12H2,1H3,(H,25,27)(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50087544
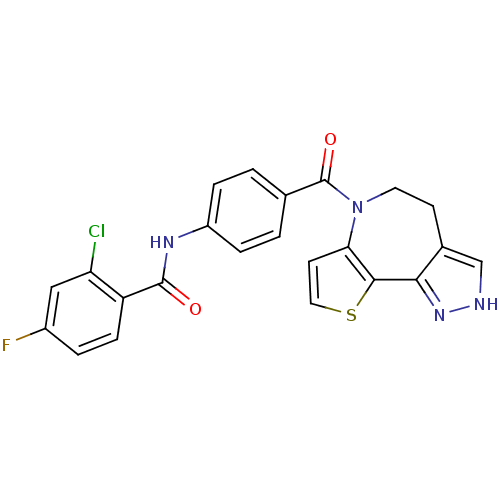
(2-Chloro-N-[4-(4,5-dihydro-2H-9-thia-1,2,6-triaza-...)Show SMILES Fc1ccc(C(=O)Nc2ccc(cc2)C(=O)N2CCc3c[nH]nc3-c3sccc23)c(Cl)c1 Show InChI InChI=1S/C23H16ClFN4O2S/c24-18-11-15(25)3-6-17(18)22(30)27-16-4-1-13(2-5-16)23(31)29-9-7-14-12-26-28-20(14)21-19(29)8-10-32-21/h1-6,8,10-12H,7,9H2,(H,26,28)(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50087545

(2,4-Dichloro-N-[4-(2-methyl-4,5-dihydro-2H-9-thia-...)Show SMILES Cn1cc2CCN(C(=O)c3ccc(NC(=O)c4ccc(Cl)cc4Cl)cc3)c3ccsc3-c2n1 Show InChI InChI=1S/C24H18Cl2N4O2S/c1-29-13-15-8-10-30(20-9-11-33-22(20)21(15)28-29)24(32)14-2-5-17(6-3-14)27-23(31)18-7-4-16(25)12-19(18)26/h2-7,9,11-13H,8,10H2,1H3,(H,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50087547

(2-Methyl-N-[4-(2-methyl-4,5-dihydro-2H-9-thia-1,2,...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCc2cn(C)nc2-c2sccc12 Show InChI InChI=1S/C25H22N4O2S/c1-16-5-3-4-6-20(16)24(30)26-19-9-7-17(8-10-19)25(31)29-13-11-18-15-28(2)27-22(18)23-21(29)12-14-32-23/h3-10,12,14-15H,11,13H2,1-2H3,(H,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50087549

(2,4-Dichloro-N-[4-(2-chloro-5,6,7,8-tetrahydro-thi...)Show SMILES Clc1cc2N(CCCCc2s1)C(=O)c1ccc(NC(=O)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C22H17Cl3N2O2S/c23-14-6-9-16(17(24)11-14)21(28)26-15-7-4-13(5-8-15)22(29)27-10-2-1-3-19-18(27)12-20(25)30-19/h4-9,11-12H,1-3,10H2,(H,26,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity for rat V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087546

(CHEMBL160029 | Cyclohexanecarboxylic acid [4-(2-me...)Show SMILES Cn1cc2CCN(C(=O)c3ccc(NC(=O)C4CCCCC4)cc3)c3ccsc3-c2n1 Show InChI InChI=1S/C24H26N4O2S/c1-27-15-18-11-13-28(20-12-14-31-22(20)21(18)26-27)24(30)17-7-9-19(10-8-17)25-23(29)16-5-3-2-4-6-16/h7-10,12,14-16H,2-6,11,13H2,1H3,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50065115

(3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C27H21ClFN3O2/c1-17-8-9-19(29)13-23(17)26(33)30-20-10-11-22(24(28)14-20)27(34)32-16-21-6-4-12-31(21)15-18-5-2-3-7-25(18)32/h2-14H,15-16H2,1H3,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50087548

(CHEMBL350383 | N-[4-(4,5-Dihydro-2H-9-thia-1,2,6-t...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCc2c[nH]nc2-c2sccc12 Show InChI InChI=1S/C24H20N4O2S/c1-15-4-2-3-5-19(15)23(29)26-18-8-6-16(7-9-18)24(30)28-12-10-17-14-25-27-21(17)22-20(28)11-13-31-22/h2-9,11,13-14H,10,12H2,1H3,(H,25,27)(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50065115

(3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(C(=O)N2Cc3cccn3Cc3ccccc23)c(Cl)c1 Show InChI InChI=1S/C27H21ClFN3O2/c1-17-8-9-19(29)13-23(17)26(33)30-20-10-11-22(24(28)14-20)27(34)32-16-21-6-4-12-31(21)15-18-5-2-3-7-25(18)32/h2-14H,15-16H2,1H3,(H,30,33) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity for rat V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50087544

(2-Chloro-N-[4-(4,5-dihydro-2H-9-thia-1,2,6-triaza-...)Show SMILES Fc1ccc(C(=O)Nc2ccc(cc2)C(=O)N2CCc3c[nH]nc3-c3sccc23)c(Cl)c1 Show InChI InChI=1S/C23H16ClFN4O2S/c24-18-11-15(25)3-6-17(18)22(30)27-16-4-1-13(2-5-16)23(31)29-9-7-14-12-26-28-20(14)21-19(29)8-10-32-21/h1-6,8,10-12H,7,9H2,(H,26,28)(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro for its binding affinity to rat V2 receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50087541

(Biphenyl-2-carboxylic acid [4-(5H,11H-benzo[e]pyrr...)Show SMILES Clc1cc(NC(=O)c2ccccc2-c2ccccc2)ccc1C(=O)N1Cc2cccn2Cc2ccccc12 Show InChI InChI=1S/C32H24ClN3O2/c33-29-19-24(34-31(37)27-14-6-5-13-26(27)22-9-2-1-3-10-22)16-17-28(29)32(38)36-21-25-12-8-18-35(25)20-23-11-4-7-15-30(23)36/h1-19H,20-21H2,(H,34,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50087555

(CHEMBL350090 | N-[4-(4,5-Dihydro-2H-9-thia-1,2,6-t...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(cc1)C(=O)N1CCc2c[nH]nc2-c2sccc12 Show InChI InChI=1S/C24H19FN4O2S/c1-14-2-5-17(25)12-19(14)23(30)27-18-6-3-15(4-7-18)24(31)29-10-8-16-13-26-28-21(16)22-20(29)9-11-32-22/h2-7,9,11-13H,8,10H2,1H3,(H,26,28)(H,27,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity for rat V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50087547

(2-Methyl-N-[4-(2-methyl-4,5-dihydro-2H-9-thia-1,2,...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCc2cn(C)nc2-c2sccc12 Show InChI InChI=1S/C25H22N4O2S/c1-16-5-3-4-6-20(16)24(30)26-19-9-7-17(8-10-19)25(31)29-13-11-18-15-28(2)27-22(18)23-21(29)12-14-32-23/h3-10,12,14-15H,11,13H2,1-2H3,(H,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50087544

(2-Chloro-N-[4-(4,5-dihydro-2H-9-thia-1,2,6-triaza-...)Show SMILES Fc1ccc(C(=O)Nc2ccc(cc2)C(=O)N2CCc3c[nH]nc3-c3sccc23)c(Cl)c1 Show InChI InChI=1S/C23H16ClFN4O2S/c24-18-11-15(25)3-6-17(18)22(30)27-16-4-1-13(2-5-16)23(31)29-9-7-14-12-26-28-20(14)21-19(29)8-10-32-21/h1-6,8,10-12H,7,9H2,(H,26,28)(H,27,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity for rat V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50087547

(2-Methyl-N-[4-(2-methyl-4,5-dihydro-2H-9-thia-1,2,...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCc2cn(C)nc2-c2sccc12 Show InChI InChI=1S/C25H22N4O2S/c1-16-5-3-4-6-20(16)24(30)26-19-9-7-17(8-10-19)25(31)29-13-11-18-15-28(2)27-22(18)23-21(29)12-14-32-23/h3-10,12,14-15H,11,13H2,1-2H3,(H,26,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity for rat V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50087548

(CHEMBL350383 | N-[4-(4,5-Dihydro-2H-9-thia-1,2,6-t...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCc2c[nH]nc2-c2sccc12 Show InChI InChI=1S/C24H20N4O2S/c1-15-4-2-3-5-19(15)23(29)26-18-8-6-16(7-9-18)24(30)28-12-10-17-14-25-27-21(17)22-20(28)11-13-31-22/h2-9,11,13-14H,10,12H2,1H3,(H,25,27)(H,26,29) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity for rat V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50087545

(2,4-Dichloro-N-[4-(2-methyl-4,5-dihydro-2H-9-thia-...)Show SMILES Cn1cc2CCN(C(=O)c3ccc(NC(=O)c4ccc(Cl)cc4Cl)cc3)c3ccsc3-c2n1 Show InChI InChI=1S/C24H18Cl2N4O2S/c1-29-13-15-8-10-30(20-9-11-33-22(20)21(15)28-29)24(32)14-2-5-17(6-3-14)27-23(31)18-7-4-16(25)12-19(18)26/h2-7,9,11-13H,8,10H2,1H3,(H,27,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity for rat V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50087545

(2,4-Dichloro-N-[4-(2-methyl-4,5-dihydro-2H-9-thia-...)Show SMILES Cn1cc2CCN(C(=O)c3ccc(NC(=O)c4ccc(Cl)cc4Cl)cc3)c3ccsc3-c2n1 Show InChI InChI=1S/C24H18Cl2N4O2S/c1-29-13-15-8-10-30(20-9-11-33-22(20)21(15)28-29)24(32)14-2-5-17(6-3-14)27-23(31)18-7-4-16(25)12-19(18)26/h2-7,9,11-13H,8,10H2,1H3,(H,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity to human V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50087546

(CHEMBL160029 | Cyclohexanecarboxylic acid [4-(2-me...)Show SMILES Cn1cc2CCN(C(=O)c3ccc(NC(=O)C4CCCCC4)cc3)c3ccsc3-c2n1 Show InChI InChI=1S/C24H26N4O2S/c1-27-15-18-11-13-28(20-12-14-31-22(20)21(18)26-27)24(30)17-7-9-19(10-8-17)25-23(29)16-5-3-2-4-6-16/h7-10,12,14-16H,2-6,11,13H2,1H3,(H,25,29) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity for rat V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50087554

(CHEMBL162748 | N-[3-Chloro-4-(4,5-dihydro-2H-9-thi...)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(C(=O)N2CCc3c[nH]nc3-c3sccc23)c(Cl)c1 Show InChI InChI=1S/C24H18ClFN4O2S/c1-13-2-3-15(26)10-18(13)23(31)28-16-4-5-17(19(25)11-16)24(32)30-8-6-14-12-27-29-21(14)22-20(30)7-9-33-22/h2-5,7,9-12H,6,8H2,1H3,(H,27,29)(H,28,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity for rat V1a receptor |
Bioorg Med Chem Lett 10: 695-8 (2000)
BindingDB Entry DOI: 10.7270/Q2X34WP8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data