 Found 53 hits Enz. Inhib. hit(s) with all data for entry = 50011994
Found 53 hits Enz. Inhib. hit(s) with all data for entry = 50011994 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM23334

(3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)OCCCc1cccnc1 |r| Show InChI InChI=1S/C20H28N2O4/c1-4-20(2,3)17(23)18(24)22-12-6-10-16(22)19(25)26-13-7-9-15-8-5-11-21-14-15/h5,8,11,14,16H,4,6-7,9-10,12-13H2,1-3H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113103
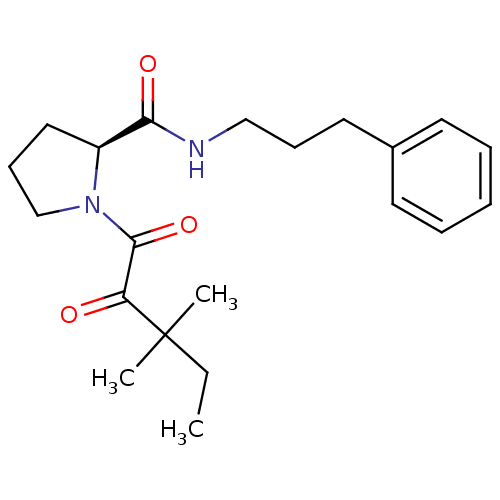
(1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCC[C@H]1C(=O)NCCCc1ccccc1 Show InChI InChI=1S/C21H30N2O3/c1-4-21(2,3)18(24)20(26)23-15-9-13-17(23)19(25)22-14-8-12-16-10-6-5-7-11-16/h5-7,10-11,17H,4,8-9,12-15H2,1-3H3,(H,22,25)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113113

((S)-1-Phenylmethanesulfonyl-piperidine-2-carboxyli...)Show SMILES O=C(NCCCCc1ccccc1)[C@@H]1CCCCN1S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C23H30N2O3S/c26-23(24-17-9-7-13-20-11-3-1-4-12-20)22-16-8-10-18-25(22)29(27,28)19-21-14-5-2-6-15-21/h1-6,11-12,14-15,22H,7-10,13,16-19H2,(H,24,26)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113133
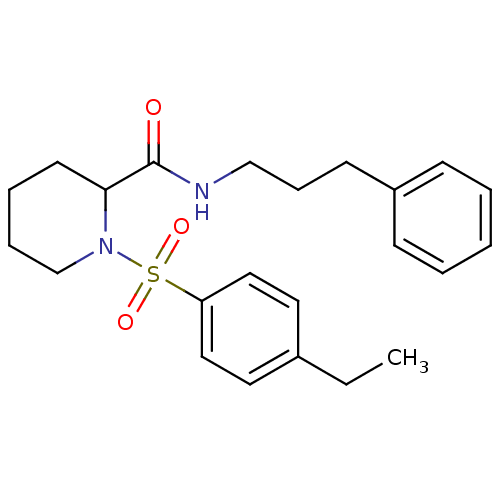
(1-(4-Ethyl-benzenesulfonyl)-piperidine-2-carboxyli...)Show SMILES CCc1ccc(cc1)S(=O)(=O)N1CCCCC1C(=O)NCCCc1ccccc1 Show InChI InChI=1S/C23H30N2O3S/c1-2-19-13-15-21(16-14-19)29(27,28)25-18-7-6-12-22(25)23(26)24-17-8-11-20-9-4-3-5-10-20/h3-5,9-10,13-16,22H,2,6-8,11-12,17-18H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113128
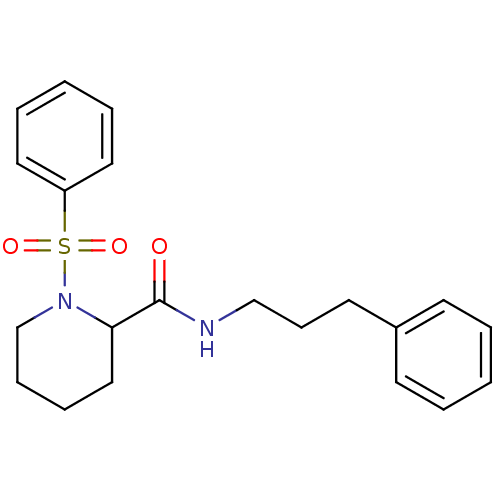
(1-Benzenesulfonyl-piperidine-2-carboxylic acid (3-...)Show InChI InChI=1S/C21H26N2O3S/c24-21(22-16-9-12-18-10-3-1-4-11-18)20-15-7-8-17-23(20)27(25,26)19-13-5-2-6-14-19/h1-6,10-11,13-14,20H,7-9,12,15-17H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113102
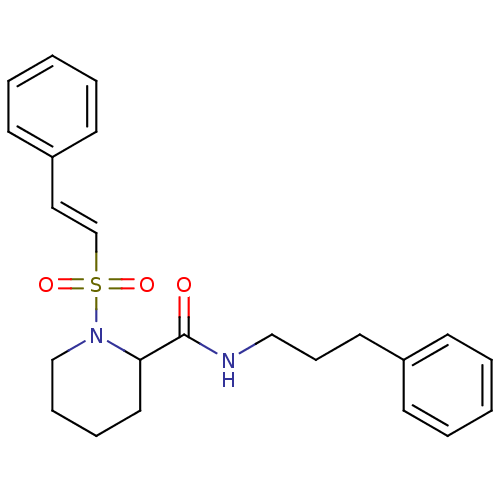
(1-((E)-2-Phenyl-ethenesulfonyl)-piperidine-2-carbo...)Show SMILES O=C(NCCCc1ccccc1)C1CCCCN1S(=O)(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C23H28N2O3S/c26-23(24-17-9-14-20-10-3-1-4-11-20)22-15-7-8-18-25(22)29(27,28)19-16-21-12-5-2-6-13-21/h1-6,10-13,16,19,22H,7-9,14-15,17-18H2,(H,24,26)/b19-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113096
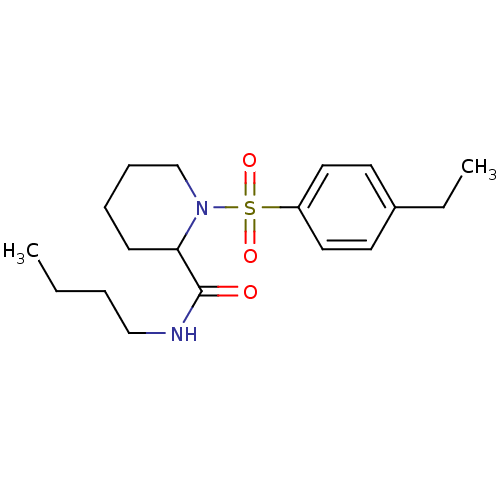
(1-(4-Ethyl-benzenesulfonyl)-piperidine-2-carboxyli...)Show InChI InChI=1S/C18H28N2O3S/c1-3-5-13-19-18(21)17-8-6-7-14-20(17)24(22,23)16-11-9-15(4-2)10-12-16/h9-12,17H,3-8,13-14H2,1-2H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113124
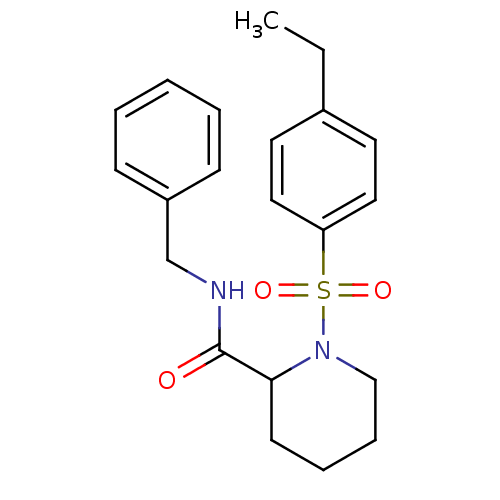
(1-(4-Ethyl-benzenesulfonyl)-piperidine-2-carboxyli...)Show SMILES CCc1ccc(cc1)S(=O)(=O)N1CCCCC1C(=O)NCc1ccccc1 Show InChI InChI=1S/C21H26N2O3S/c1-2-17-11-13-19(14-12-17)27(25,26)23-15-7-6-10-20(23)21(24)22-16-18-8-4-3-5-9-18/h3-5,8-9,11-14,20H,2,6-7,10,15-16H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113123
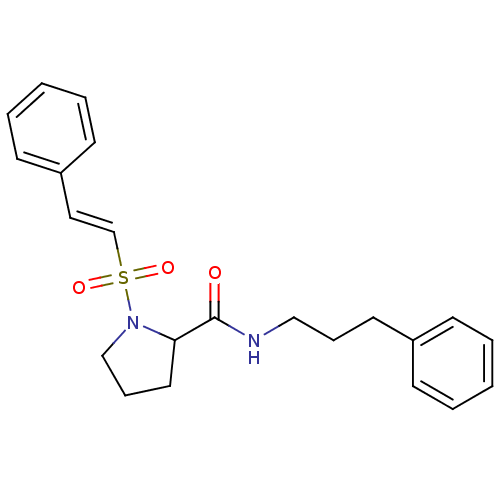
(1-((E)-2-Phenyl-ethenesulfonyl)-pyrrolidine-2-carb...)Show SMILES O=C(NCCCc1ccccc1)C1CCCN1S(=O)(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C22H26N2O3S/c25-22(23-16-7-13-19-9-3-1-4-10-19)21-14-8-17-24(21)28(26,27)18-15-20-11-5-2-6-12-20/h1-6,9-12,15,18,21H,7-8,13-14,16-17H2,(H,23,25)/b18-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113142
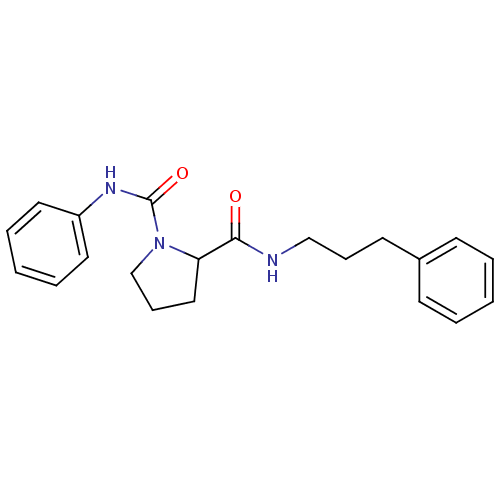
(CHEMBL33817 | Pyrrolidine-1,2-dicarboxylic acid 1-...)Show InChI InChI=1S/C21H25N3O2/c25-20(22-15-7-11-17-9-3-1-4-10-17)19-14-8-16-24(19)21(26)23-18-12-5-2-6-13-18/h1-6,9-10,12-13,19H,7-8,11,14-16H2,(H,22,25)(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113130
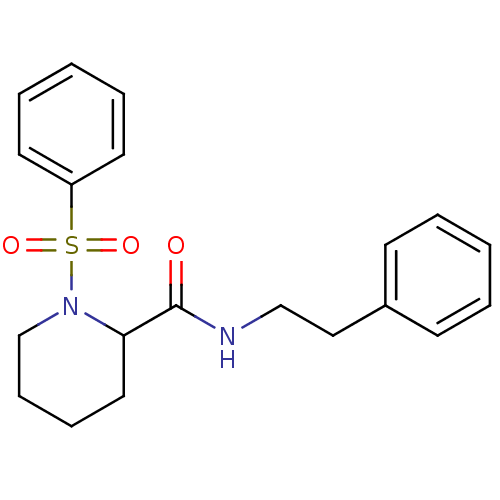
(1-Benzenesulfonyl-piperidine-2-carboxylic acid phe...)Show InChI InChI=1S/C20H24N2O3S/c23-20(21-15-14-17-9-3-1-4-10-17)19-13-7-8-16-22(19)26(24,25)18-11-5-2-6-12-18/h1-6,9-12,19H,7-8,13-16H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113145
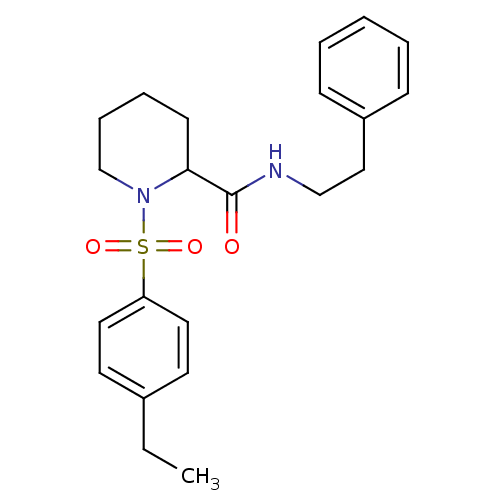
(1-(4-Ethyl-benzenesulfonyl)-piperidine-2-carboxyli...)Show SMILES CCc1ccc(cc1)S(=O)(=O)N1CCCCC1C(=O)NCCc1ccccc1 Show InChI InChI=1S/C22H28N2O3S/c1-2-18-11-13-20(14-12-18)28(26,27)24-17-7-6-10-21(24)22(25)23-16-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,21H,2,6-7,10,15-17H2,1H3,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113109
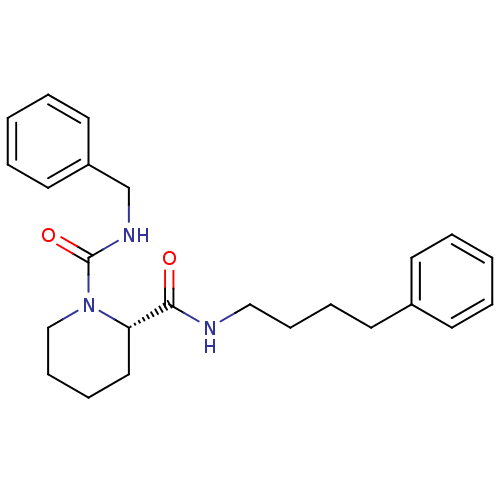
((S)-Piperidine-1,2-dicarboxylic acid 1-benzylamide...)Show SMILES O=C(NCCCCc1ccccc1)[C@@H]1CCCCN1C(=O)NCc1ccccc1 Show InChI InChI=1S/C24H31N3O2/c28-23(25-17-9-7-13-20-11-3-1-4-12-20)22-16-8-10-18-27(22)24(29)26-19-21-14-5-2-6-15-21/h1-6,11-12,14-15,22H,7-10,13,16-19H2,(H,25,28)(H,26,29)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113104
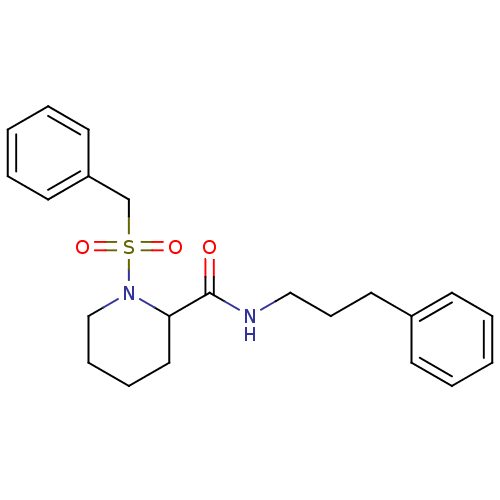
(1-Phenylmethanesulfonyl-piperidine-2-carboxylic ac...)Show SMILES O=C(NCCCc1ccccc1)C1CCCCN1S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C22H28N2O3S/c25-22(23-16-9-14-19-10-3-1-4-11-19)21-15-7-8-17-24(21)28(26,27)18-20-12-5-2-6-13-20/h1-6,10-13,21H,7-9,14-18H2,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113147
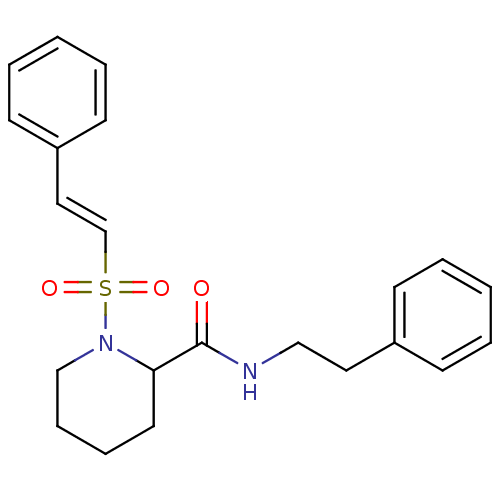
(1-((E)-2-Phenyl-ethenesulfonyl)-piperidine-2-carbo...)Show SMILES O=C(NCCc1ccccc1)C1CCCCN1S(=O)(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C22H26N2O3S/c25-22(23-16-14-19-9-3-1-4-10-19)21-13-7-8-17-24(21)28(26,27)18-15-20-11-5-2-6-12-20/h1-6,9-12,15,18,21H,7-8,13-14,16-17H2,(H,23,25)/b18-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113126
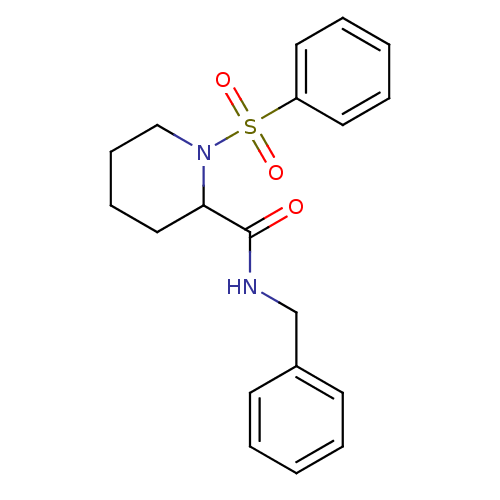
(1-Benzenesulfonyl-piperidine-2-carboxylic acid ben...)Show InChI InChI=1S/C19H22N2O3S/c22-19(20-15-16-9-3-1-4-10-16)18-13-7-8-14-21(18)25(23,24)17-11-5-2-6-12-17/h1-6,9-12,18H,7-8,13-15H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113100
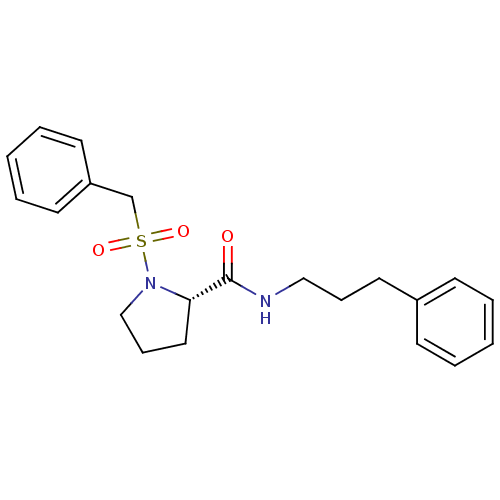
((S)-1-Phenylmethanesulfonyl-pyrrolidine-2-carboxyl...)Show SMILES O=C(NCCCc1ccccc1)[C@@H]1CCCN1S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C21H26N2O3S/c24-21(22-15-7-13-18-9-3-1-4-10-18)20-14-8-16-23(20)27(25,26)17-19-11-5-2-6-12-19/h1-6,9-12,20H,7-8,13-17H2,(H,22,24)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113117
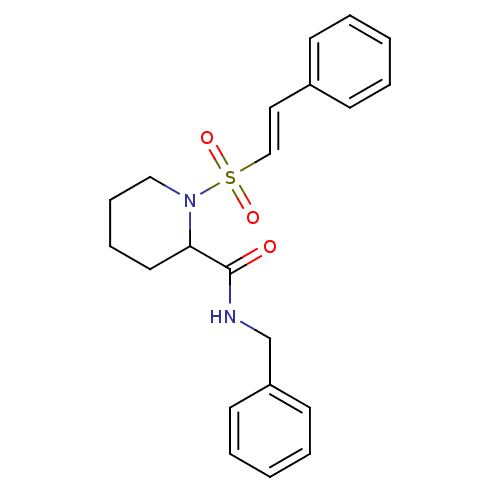
(1-((E)-2-Phenyl-ethenesulfonyl)-piperidine-2-carbo...)Show SMILES O=C(NCc1ccccc1)C1CCCCN1S(=O)(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C21H24N2O3S/c24-21(22-17-19-11-5-2-6-12-19)20-13-7-8-15-23(20)27(25,26)16-14-18-9-3-1-4-10-18/h1-6,9-12,14,16,20H,7-8,13,15,17H2,(H,22,24)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113137
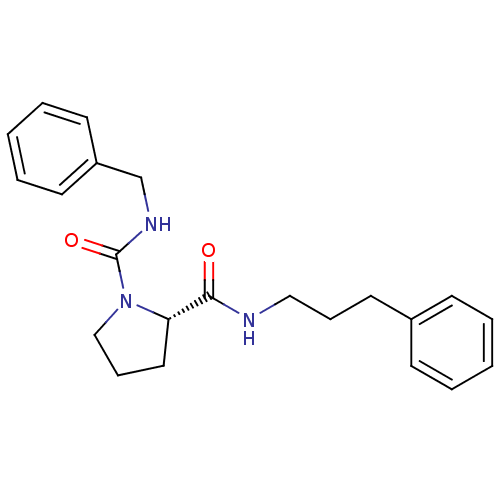
((S)-Pyrrolidine-1,2-dicarboxylic acid 1-benzylamid...)Show SMILES O=C(NCCCc1ccccc1)[C@@H]1CCCN1C(=O)NCc1ccccc1 Show InChI InChI=1S/C22H27N3O2/c26-21(23-15-7-13-18-9-3-1-4-10-18)20-14-8-16-25(20)22(27)24-17-19-11-5-2-6-12-19/h1-6,9-12,20H,7-8,13-17H2,(H,23,26)(H,24,27)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113144

(1-Benzenesulfonyl-piperidine-2-carboxylic acid but...)Show InChI InChI=1S/C16H24N2O3S/c1-2-3-12-17-16(19)15-11-7-8-13-18(15)22(20,21)14-9-5-4-6-10-14/h4-6,9-10,15H,2-3,7-8,11-13H2,1H3,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113099
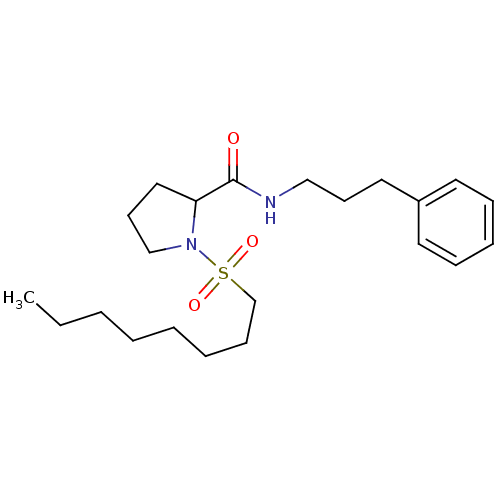
(1-(Octane-1-sulfonyl)-pyrrolidine-2-carboxylic aci...)Show InChI InChI=1S/C22H36N2O3S/c1-2-3-4-5-6-10-19-28(26,27)24-18-12-16-21(24)22(25)23-17-11-15-20-13-8-7-9-14-20/h7-9,13-14,21H,2-6,10-12,15-19H2,1H3,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113138

(1-(Toluene-4-sulfonyl)-pyrrolidine-2-carboxylic ac...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCCC1C(=O)NCCCc1ccccc1 Show InChI InChI=1S/C21H26N2O3S/c1-17-11-13-19(14-12-17)27(25,26)23-16-6-10-20(23)21(24)22-15-5-9-18-7-3-2-4-8-18/h2-4,7-8,11-14,20H,5-6,9-10,15-16H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113116
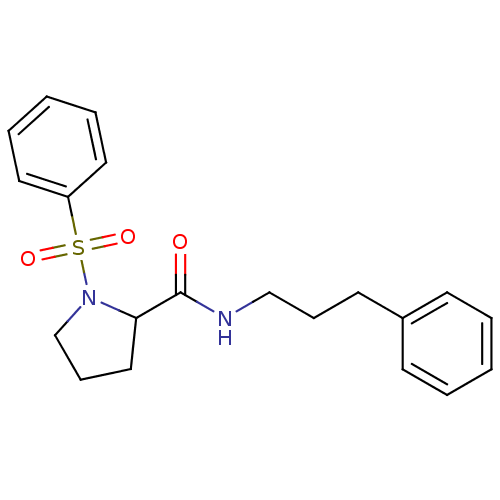
(1-Benzenesulfonyl-pyrrolidine-2-carboxylic acid (3...)Show InChI InChI=1S/C20H24N2O3S/c23-20(21-15-7-11-17-9-3-1-4-10-17)19-14-8-16-22(19)26(24,25)18-12-5-2-6-13-18/h1-6,9-10,12-13,19H,7-8,11,14-16H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113136
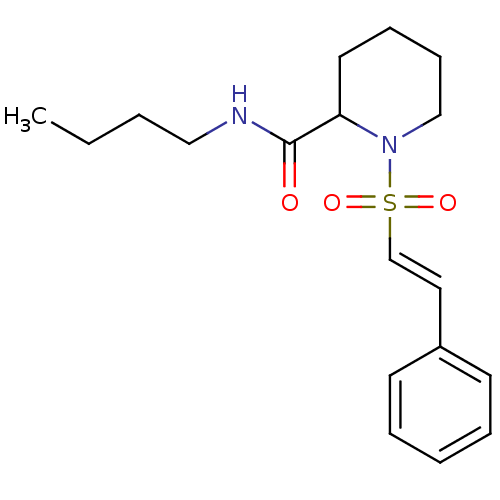
(1-((E)-2-Phenyl-ethenesulfonyl)-piperidine-2-carbo...)Show InChI InChI=1S/C18H26N2O3S/c1-2-3-13-19-18(21)17-11-7-8-14-20(17)24(22,23)15-12-16-9-5-4-6-10-16/h4-6,9-10,12,15,17H,2-3,7-8,11,13-14H2,1H3,(H,19,21)/b15-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113120
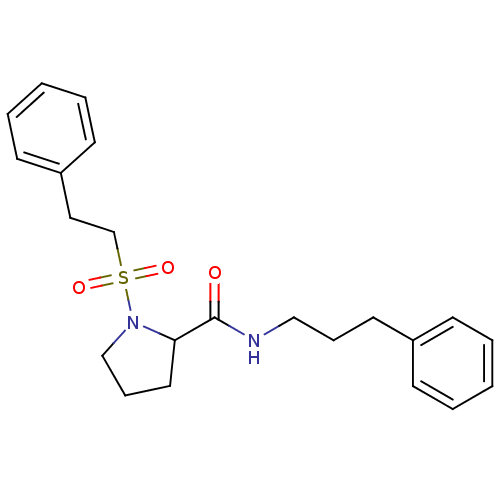
(1-(2-Phenyl-ethanesulfonyl)-pyrrolidine-2-carboxyl...)Show SMILES O=C(NCCCc1ccccc1)C1CCCN1S(=O)(=O)CCc1ccccc1 Show InChI InChI=1S/C22H28N2O3S/c25-22(23-16-7-13-19-9-3-1-4-10-19)21-14-8-17-24(21)28(26,27)18-15-20-11-5-2-6-12-20/h1-6,9-12,21H,7-8,13-18H2,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113101
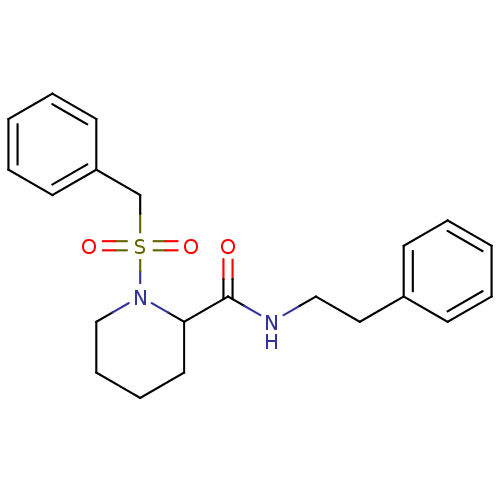
(1-Phenylmethanesulfonyl-piperidine-2-carboxylic ac...)Show InChI InChI=1S/C21H26N2O3S/c24-21(22-15-14-18-9-3-1-4-10-18)20-13-7-8-16-23(20)27(25,26)17-19-11-5-2-6-12-19/h1-6,9-12,20H,7-8,13-17H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113135
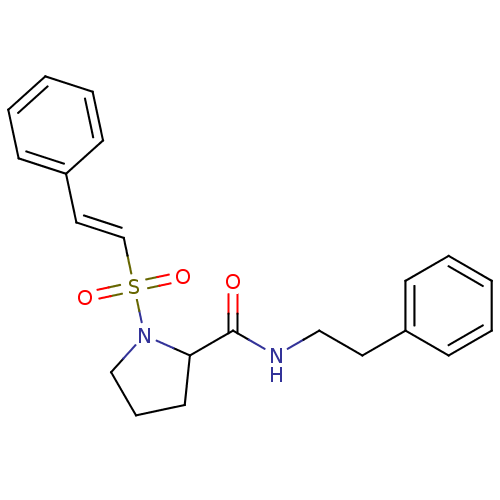
(1-((E)-2-Phenyl-ethenesulfonyl)-pyrrolidine-2-carb...)Show SMILES O=C(NCCc1ccccc1)C1CCCN1S(=O)(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C21H24N2O3S/c24-21(22-15-13-18-8-3-1-4-9-18)20-12-7-16-23(20)27(25,26)17-14-19-10-5-2-6-11-19/h1-6,8-11,14,17,20H,7,12-13,15-16H2,(H,22,24)/b17-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113105
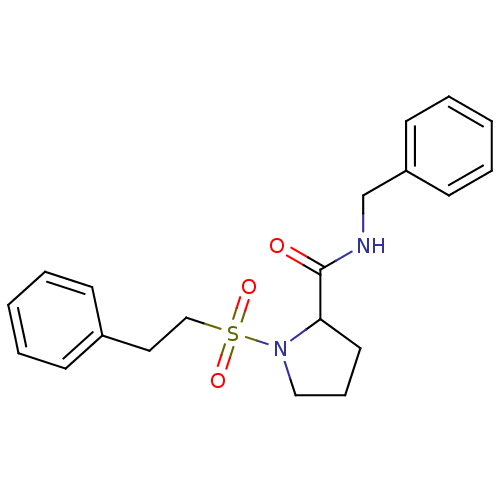
(1-(2-Phenyl-ethanesulfonyl)-pyrrolidine-2-carboxyl...)Show InChI InChI=1S/C20H24N2O3S/c23-20(21-16-18-10-5-2-6-11-18)19-12-7-14-22(19)26(24,25)15-13-17-8-3-1-4-9-17/h1-6,8-11,19H,7,12-16H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113146
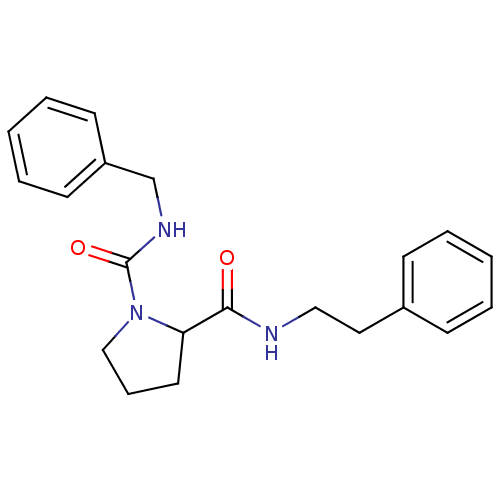
(CHEMBL285034 | Pyrrolidine-1,2-dicarboxylic acid 1...)Show InChI InChI=1S/C21H25N3O2/c25-20(22-14-13-17-8-3-1-4-9-17)19-12-7-15-24(19)21(26)23-16-18-10-5-2-6-11-18/h1-6,8-11,19H,7,12-16H2,(H,22,25)(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113131
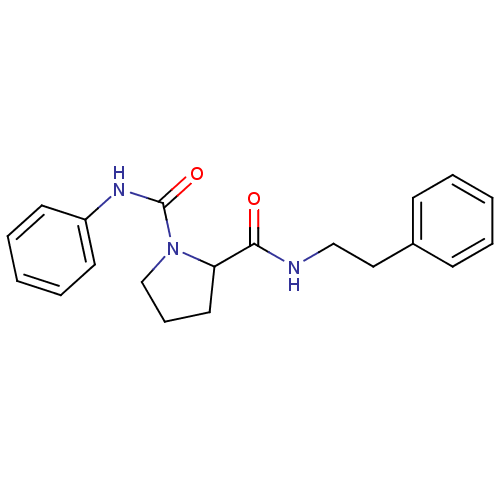
(CHEMBL33413 | Pyrrolidine-1,2-dicarboxylic acid 2-...)Show InChI InChI=1S/C20H23N3O2/c24-19(21-14-13-16-8-3-1-4-9-16)18-12-7-15-23(18)20(25)22-17-10-5-2-6-11-17/h1-6,8-11,18H,7,12-15H2,(H,21,24)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113121
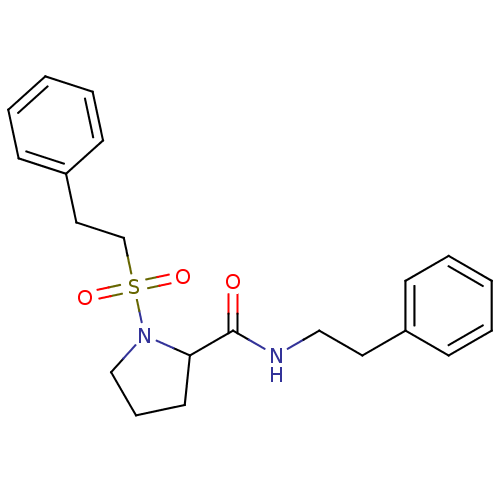
(1-(2-Phenyl-ethanesulfonyl)-pyrrolidine-2-carboxyl...)Show InChI InChI=1S/C21H26N2O3S/c24-21(22-15-13-18-8-3-1-4-9-18)20-12-7-16-23(20)27(25,26)17-14-19-10-5-2-6-11-19/h1-6,8-11,20H,7,12-17H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113108

(1-(Toluene-4-sulfonyl)-pyrrolidine-2-carboxylic ac...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCCC1C(=O)NCCc1ccccc1 Show InChI InChI=1S/C20H24N2O3S/c1-16-9-11-18(12-10-16)26(24,25)22-15-5-8-19(22)20(23)21-14-13-17-6-3-2-4-7-17/h2-4,6-7,9-12,19H,5,8,13-15H2,1H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113127
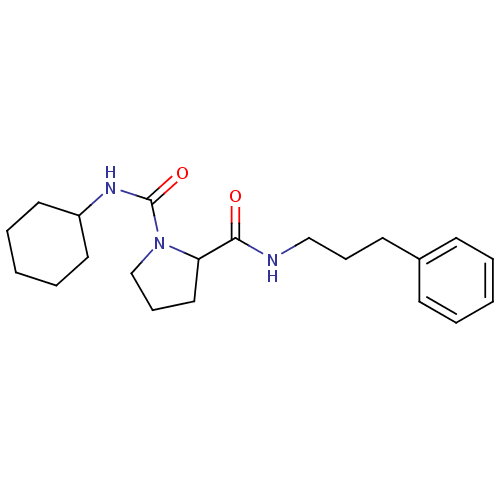
(CHEMBL33728 | Pyrrolidine-1,2-dicarboxylic acid 1-...)Show InChI InChI=1S/C21H31N3O2/c25-20(22-15-7-11-17-9-3-1-4-10-17)19-14-8-16-24(19)21(26)23-18-12-5-2-6-13-18/h1,3-4,9-10,18-19H,2,5-8,11-16H2,(H,22,25)(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113139
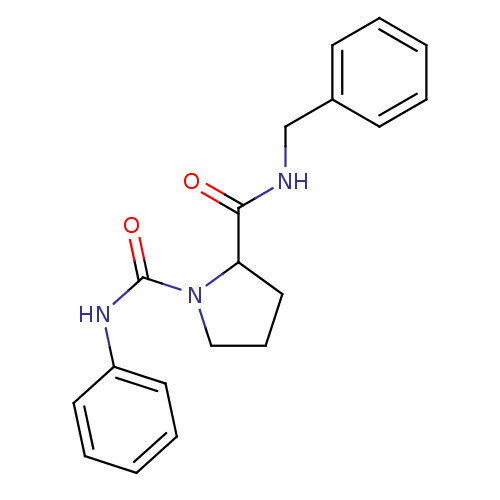
(CHEMBL34746 | Pyrrolidine-1,2-dicarboxylic acid 2-...)Show InChI InChI=1S/C19H21N3O2/c23-18(20-14-15-8-3-1-4-9-15)17-12-7-13-22(17)19(24)21-16-10-5-2-6-11-16/h1-6,8-11,17H,7,12-14H2,(H,20,23)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113118

(1-Benzenesulfonyl-pyrrolidine-2-carboxylic acid ph...)Show InChI InChI=1S/C19H22N2O3S/c22-19(20-14-13-16-8-3-1-4-9-16)18-12-7-15-21(18)25(23,24)17-10-5-2-6-11-17/h1-6,8-11,18H,7,12-15H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113098
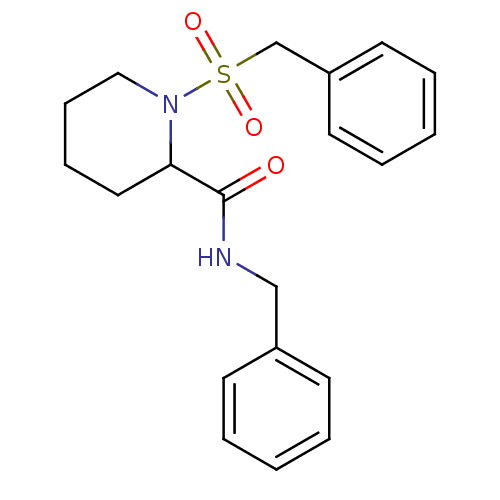
(1-Phenylmethanesulfonyl-piperidine-2-carboxylic ac...)Show InChI InChI=1S/C20H24N2O3S/c23-20(21-15-17-9-3-1-4-10-17)19-13-7-8-14-22(19)26(24,25)16-18-11-5-2-6-12-18/h1-6,9-12,19H,7-8,13-16H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113129
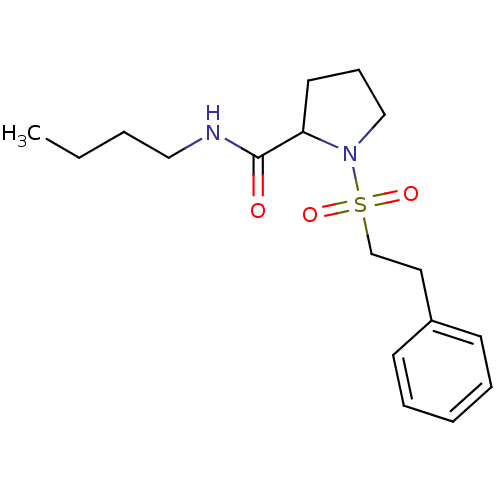
(1-(2-Phenyl-ethanesulfonyl)-pyrrolidine-2-carboxyl...)Show InChI InChI=1S/C17H26N2O3S/c1-2-3-12-18-17(20)16-10-7-13-19(16)23(21,22)14-11-15-8-5-4-6-9-15/h4-6,8-9,16H,2-3,7,10-14H2,1H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113112
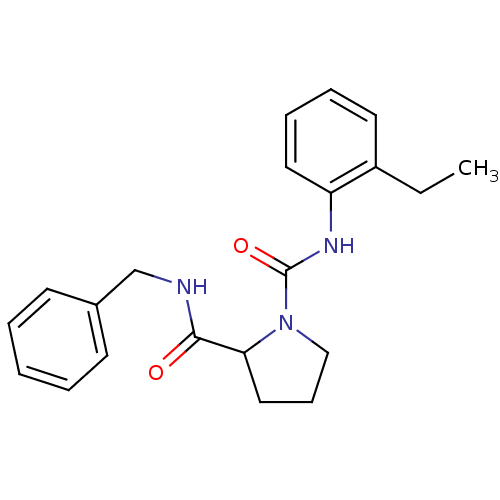
(CHEMBL33597 | Pyrrolidine-1,2-dicarboxylic acid 2-...)Show InChI InChI=1S/C21H25N3O2/c1-2-17-11-6-7-12-18(17)23-21(26)24-14-8-13-19(24)20(25)22-15-16-9-4-3-5-10-16/h3-7,9-12,19H,2,8,13-15H2,1H3,(H,22,25)(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113114
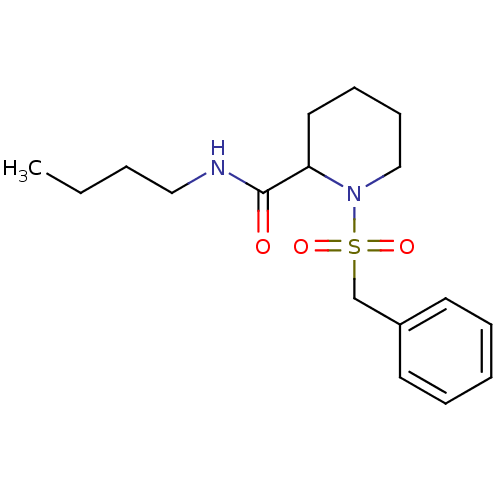
(1-Phenylmethanesulfonyl-piperidine-2-carboxylic ac...)Show InChI InChI=1S/C17H26N2O3S/c1-2-3-12-18-17(20)16-11-7-8-13-19(16)23(21,22)14-15-9-5-4-6-10-15/h4-6,9-10,16H,2-3,7-8,11-14H2,1H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113110
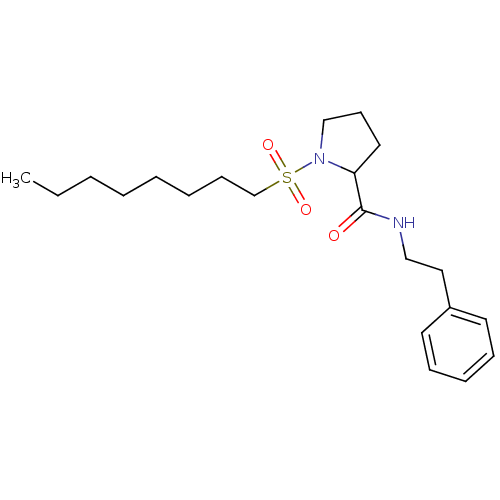
(1-(Octane-1-sulfonyl)-pyrrolidine-2-carboxylic aci...)Show InChI InChI=1S/C21H34N2O3S/c1-2-3-4-5-6-10-18-27(25,26)23-17-11-14-20(23)21(24)22-16-15-19-12-8-7-9-13-19/h7-9,12-13,20H,2-6,10-11,14-18H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113143
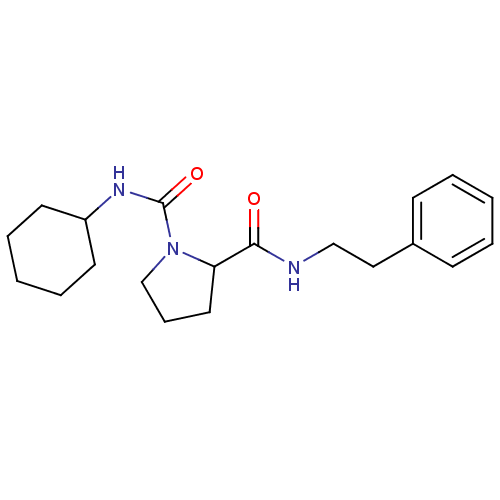
(CHEMBL34673 | Pyrrolidine-1,2-dicarboxylic acid 1-...)Show InChI InChI=1S/C20H29N3O2/c24-19(21-14-13-16-8-3-1-4-9-16)18-12-7-15-23(18)20(25)22-17-10-5-2-6-11-17/h1,3-4,8-9,17-18H,2,5-7,10-15H2,(H,21,24)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113140
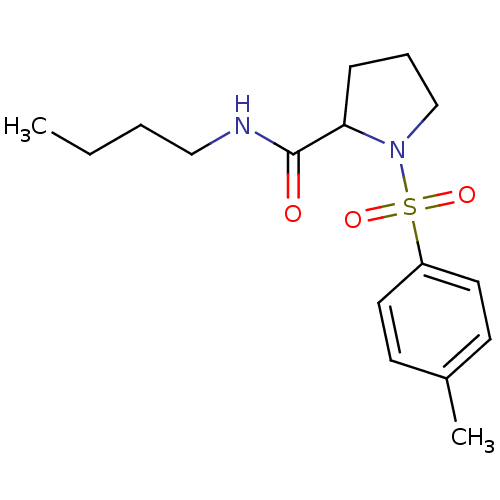
(1-(Toluene-4-sulfonyl)-pyrrolidine-2-carboxylic ac...)Show InChI InChI=1S/C16H24N2O3S/c1-3-4-11-17-16(19)15-6-5-12-18(15)22(20,21)14-9-7-13(2)8-10-14/h7-10,15H,3-6,11-12H2,1-2H3,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113141
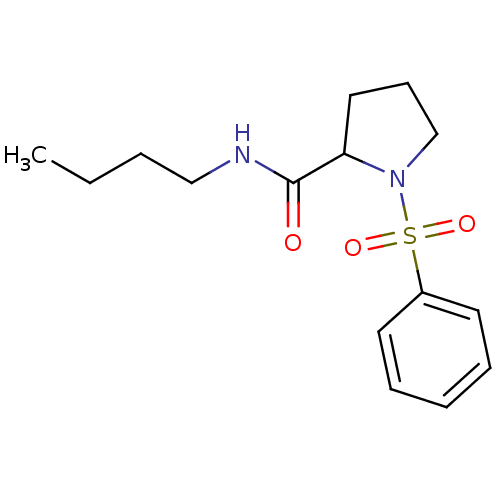
(1-Benzenesulfonyl-pyrrolidine-2-carboxylic acid bu...)Show InChI InChI=1S/C15H22N2O3S/c1-2-3-11-16-15(18)14-10-7-12-17(14)21(19,20)13-8-5-4-6-9-13/h4-6,8-9,14H,2-3,7,10-12H2,1H3,(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113106
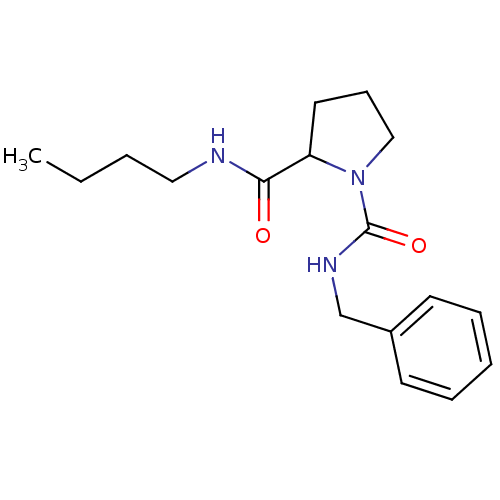
(CHEMBL32998 | Pyrrolidine-1,2-dicarboxylic acid 1-...)Show InChI InChI=1S/C17H25N3O2/c1-2-3-11-18-16(21)15-10-7-12-20(15)17(22)19-13-14-8-5-4-6-9-14/h4-6,8-9,15H,2-3,7,10-13H2,1H3,(H,18,21)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
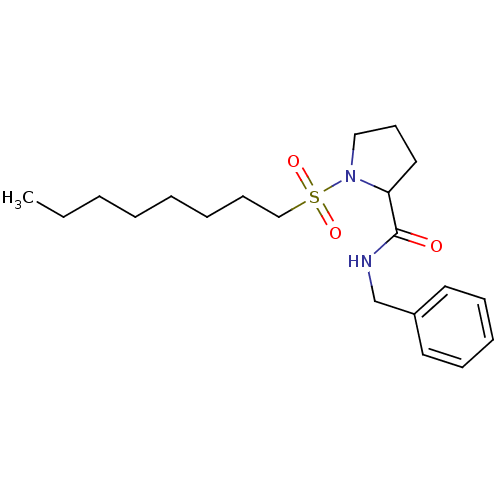
(Homo sapiens (Human)) | BDBM50113107
(1-(Octane-1-sulfonyl)-pyrrolidine-2-carboxylic aci...)Show InChI InChI=1S/C20H32N2O3S/c1-2-3-4-5-6-10-16-26(24,25)22-15-11-14-19(22)20(23)21-17-18-12-8-7-9-13-18/h7-9,12-13,19H,2-6,10-11,14-17H2,1H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113132
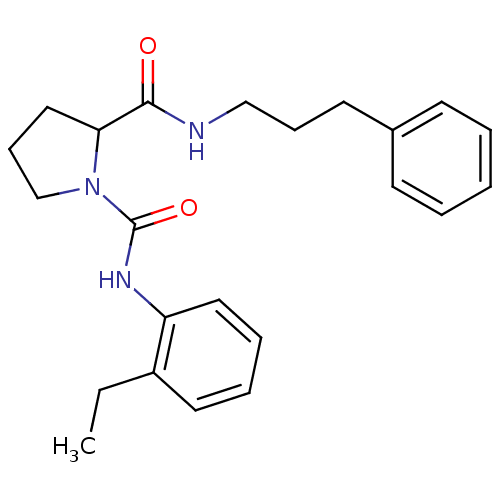
(CHEMBL34559 | Pyrrolidine-1,2-dicarboxylic acid 1-...)Show InChI InChI=1S/C23H29N3O2/c1-2-19-13-6-7-14-20(19)25-23(28)26-17-9-15-21(26)22(27)24-16-8-12-18-10-4-3-5-11-18/h3-7,10-11,13-14,21H,2,8-9,12,15-17H2,1H3,(H,24,27)(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113097
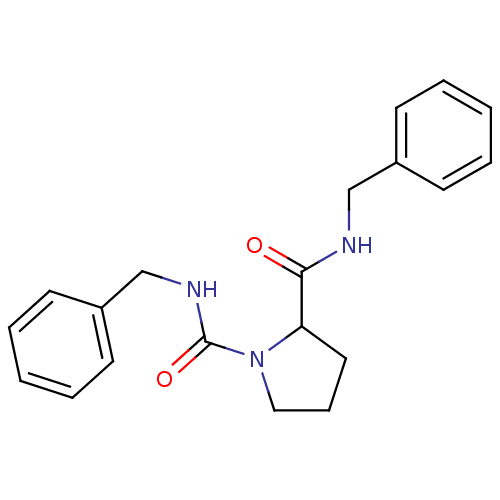
(CHEMBL32414 | Pyrrolidine-1,2-dicarboxylic acid bi...)Show InChI InChI=1S/C20H23N3O2/c24-19(21-14-16-8-3-1-4-9-16)18-12-7-13-23(18)20(25)22-15-17-10-5-2-6-11-17/h1-6,8-11,18H,7,12-15H2,(H,21,24)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113111
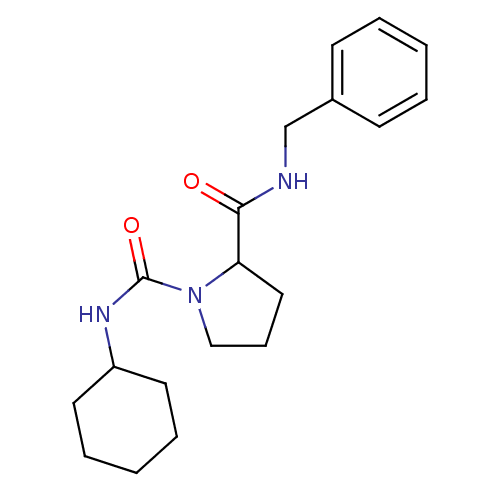
(CHEMBL34060 | Pyrrolidine-1,2-dicarboxylic acid 2-...)Show InChI InChI=1S/C19H27N3O2/c23-18(20-14-15-8-3-1-4-9-15)17-12-7-13-22(17)19(24)21-16-10-5-2-6-11-16/h1,3-4,8-9,16-17H,2,5-7,10-14H2,(H,20,23)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113115
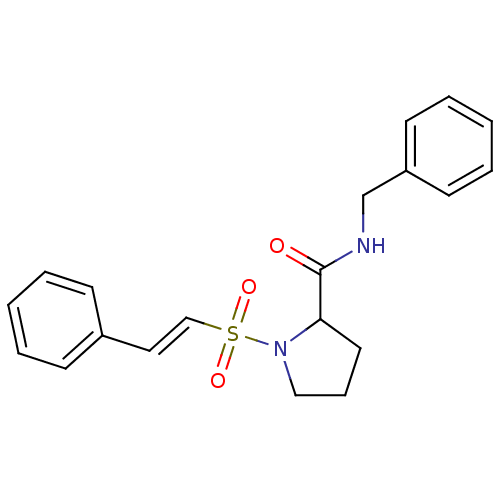
(1-((E)-2-Phenyl-ethenesulfonyl)-pyrrolidine-2-carb...)Show SMILES O=C(NCc1ccccc1)C1CCCN1S(=O)(=O)\C=C\c1ccccc1 Show InChI InChI=1S/C20H22N2O3S/c23-20(21-16-18-10-5-2-6-11-18)19-12-7-14-22(19)26(24,25)15-13-17-8-3-1-4-9-17/h1-6,8-11,13,15,19H,7,12,14,16H2,(H,21,23)/b15-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50113134
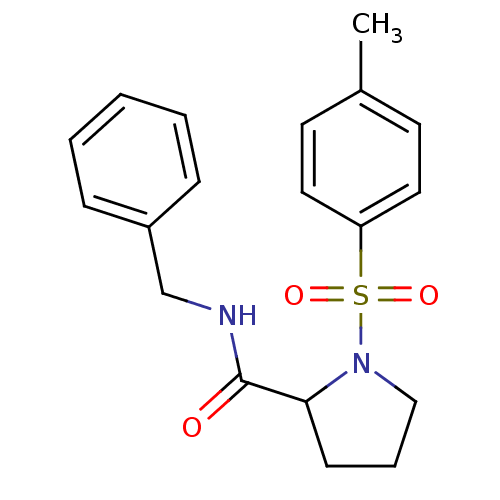
(1-(Toluene-4-sulfonyl)-pyrrolidine-2-carboxylic ac...)Show InChI InChI=1S/C19H22N2O3S/c1-15-9-11-17(12-10-15)25(23,24)21-13-5-8-18(21)19(22)20-14-16-6-3-2-4-7-16/h2-4,6-7,9-12,18H,5,8,13-14H2,1H3,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 |
Bioorg Med Chem Lett 12: 1429-33 (2002)
BindingDB Entry DOI: 10.7270/Q23J3C9S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data