 Found 63 hits Enz. Inhib. hit(s) with all data for entry = 50038255
Found 63 hits Enz. Inhib. hit(s) with all data for entry = 50038255 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231682
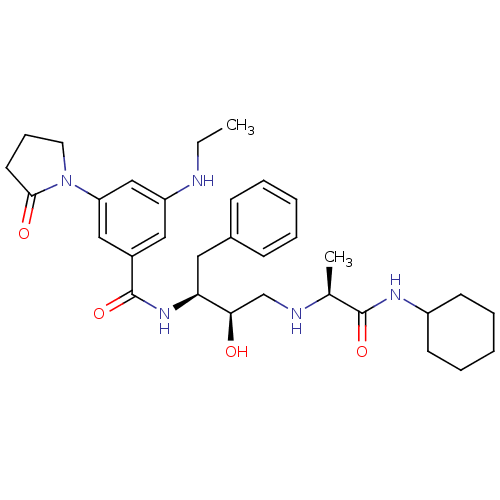
(CHEMBL251862 | N-((2S,3R)-4-((S)-1-(cyclohexylamin...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C32H45N5O4/c1-3-33-26-18-24(19-27(20-26)37-16-10-15-30(37)39)32(41)36-28(17-23-11-6-4-7-12-23)29(38)21-34-22(2)31(40)35-25-13-8-5-9-14-25/h4,6-7,11-12,18-20,22,25,28-29,33-34,38H,3,5,8-10,13-17,21H2,1-2H3,(H,35,40)(H,36,41)/t22-,28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373809
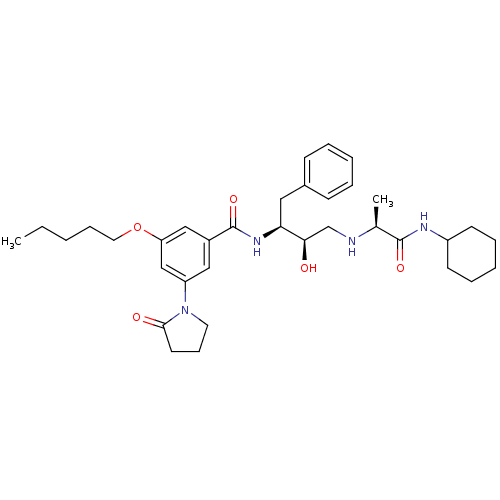
(CHEMBL256399)Show SMILES CCCCCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C35H50N4O5/c1-3-4-11-19-44-30-22-27(21-29(23-30)39-18-12-17-33(39)41)35(43)38-31(20-26-13-7-5-8-14-26)32(40)24-36-25(2)34(42)37-28-15-9-6-10-16-28/h5,7-8,13-14,21-23,25,28,31-32,36,40H,3-4,6,9-12,15-20,24H2,1-2H3,(H,37,42)(H,38,43)/t25-,31-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373806
(CHEMBL227053)Show SMILES CC(C)Nc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H47N5O4/c1-22(2)35-27-18-25(19-28(20-27)38-16-10-15-31(38)40)33(42)37-29(17-24-11-6-4-7-12-24)30(39)21-34-23(3)32(41)36-26-13-8-5-9-14-26/h4,6-7,11-12,18-20,22-23,26,29-30,34-35,39H,5,8-10,13-17,21H2,1-3H3,(H,36,41)(H,37,42)/t23-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373810
(CHEMBL258068)Show SMILES CCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C32H44N4O5/c1-3-41-27-19-24(18-26(20-27)36-16-10-15-30(36)38)32(40)35-28(17-23-11-6-4-7-12-23)29(37)21-33-22(2)31(39)34-25-13-8-5-9-14-25/h4,6-7,11-12,18-20,22,25,28-29,33,37H,3,5,8-10,13-17,21H2,1-2H3,(H,34,39)(H,35,40)/t22-,28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373804
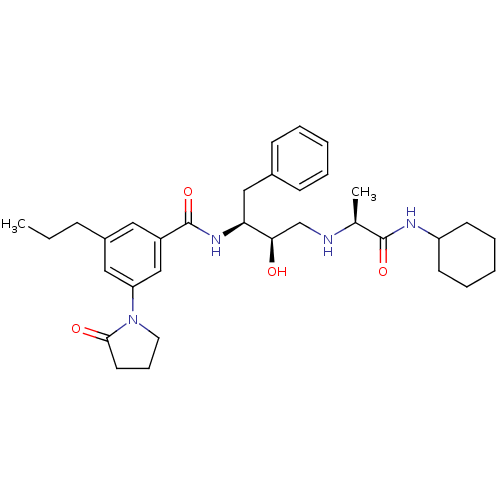
(CHEMBL257871)Show SMILES CCCc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O4/c1-3-11-25-18-26(21-28(19-25)37-17-10-16-31(37)39)33(41)36-29(20-24-12-6-4-7-13-24)30(38)22-34-23(2)32(40)35-27-14-8-5-9-15-27/h4,6-7,12-13,18-19,21,23,27,29-30,34,38H,3,5,8-11,14-17,20,22H2,1-2H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373800
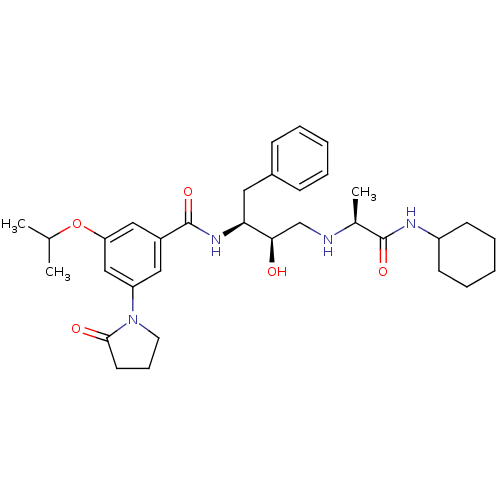
(CHEMBL255977)Show SMILES CC(C)Oc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O5/c1-22(2)42-28-19-25(18-27(20-28)37-16-10-15-31(37)39)33(41)36-29(17-24-11-6-4-7-12-24)30(38)21-34-23(3)32(40)35-26-13-8-5-9-14-26/h4,6-7,11-12,18-20,22-23,26,29-30,34,38H,5,8-10,13-17,21H2,1-3H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373805
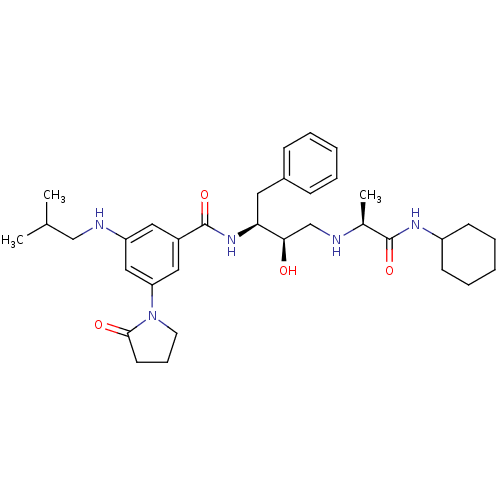
(CHEMBL255964)Show SMILES CC(C)CNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C34H49N5O4/c1-23(2)21-36-28-18-26(19-29(20-28)39-16-10-15-32(39)41)34(43)38-30(17-25-11-6-4-7-12-25)31(40)22-35-24(3)33(42)37-27-13-8-5-9-14-27/h4,6-7,11-12,18-20,23-24,27,30-31,35-36,40H,5,8-10,13-17,21-22H2,1-3H3,(H,37,42)(H,38,43)/t24-,30-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373806

(CHEMBL227053)Show SMILES CC(C)Nc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H47N5O4/c1-22(2)35-27-18-25(19-28(20-27)38-16-10-15-31(38)40)33(42)37-29(17-24-11-6-4-7-12-24)30(39)21-34-23(3)32(41)36-26-13-8-5-9-14-26/h4,6-7,11-12,18-20,22-23,26,29-30,34-35,39H,5,8-10,13-17,21H2,1-3H3,(H,36,41)(H,37,42)/t23-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in SHSY5Y cells assessed as reduction of amyloid beta 42 production |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231682

(CHEMBL251862 | N-((2S,3R)-4-((S)-1-(cyclohexylamin...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C32H45N5O4/c1-3-33-26-18-24(19-27(20-26)37-16-10-15-30(37)39)32(41)36-28(17-23-11-6-4-7-12-23)29(38)21-34-22(2)31(40)35-25-13-8-5-9-14-25/h4,6-7,11-12,18-20,22,25,28-29,33-34,38H,3,5,8-10,13-17,21H2,1-2H3,(H,35,40)(H,36,41)/t22-,28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in SHSY5Y cells assessed as reduction of amyloid beta 40 production |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373806

(CHEMBL227053)Show SMILES CC(C)Nc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H47N5O4/c1-22(2)35-27-18-25(19-28(20-27)38-16-10-15-31(38)40)33(42)37-29(17-24-11-6-4-7-12-24)30(39)21-34-23(3)32(41)36-26-13-8-5-9-14-26/h4,6-7,11-12,18-20,22-23,26,29-30,34-35,39H,5,8-10,13-17,21H2,1-3H3,(H,36,41)(H,37,42)/t23-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in SHSY5Y cells assessed as reduction of amyloid beta 40 production |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231682

(CHEMBL251862 | N-((2S,3R)-4-((S)-1-(cyclohexylamin...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C32H45N5O4/c1-3-33-26-18-24(19-27(20-26)37-16-10-15-30(37)39)32(41)36-28(17-23-11-6-4-7-12-23)29(38)21-34-22(2)31(40)35-25-13-8-5-9-14-25/h4,6-7,11-12,18-20,22,25,28-29,33-34,38H,3,5,8-10,13-17,21H2,1-2H3,(H,35,40)(H,36,41)/t22-,28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in SHSY5Y cells assessed as reduction of amyloid beta 42 production |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50373809

(CHEMBL256399)Show SMILES CCCCCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C35H50N4O5/c1-3-4-11-19-44-30-22-27(21-29(23-30)39-18-12-17-33(39)41)35(43)38-31(20-26-13-7-5-8-14-26)32(40)24-36-25(2)34(42)37-28-15-9-6-10-16-28/h5,7-8,13-14,21-23,25,28,31-32,36,40H,3-4,6,9-12,15-20,24H2,1-2H3,(H,37,42)(H,38,43)/t25-,31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50373809

(CHEMBL256399)Show SMILES CCCCCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C35H50N4O5/c1-3-4-11-19-44-30-22-27(21-29(23-30)39-18-12-17-33(39)41)35(43)38-31(20-26-13-7-5-8-14-26)32(40)24-36-25(2)34(42)37-28-15-9-6-10-16-28/h5,7-8,13-14,21-23,25,28,31-32,36,40H,3-4,6,9-12,15-20,24H2,1-2H3,(H,37,42)(H,38,43)/t25-,31-,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50373812
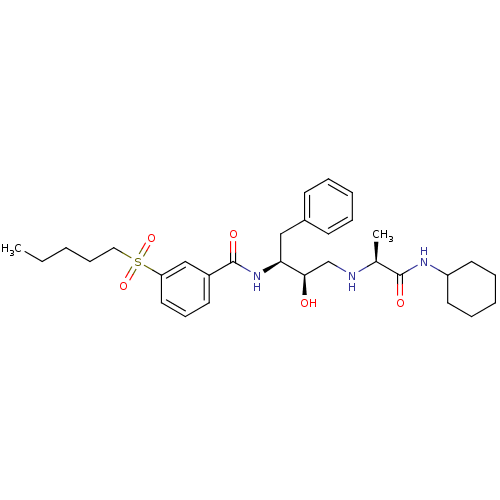
(CHEMBL402305)Show SMILES CCCCCS(=O)(=O)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C31H45N3O5S/c1-3-4-11-19-40(38,39)27-18-12-15-25(21-27)31(37)34-28(20-24-13-7-5-8-14-24)29(35)22-32-23(2)30(36)33-26-16-9-6-10-17-26/h5,7-8,12-15,18,21,23,26,28-29,32,35H,3-4,6,9-11,16-17,19-20,22H2,1-2H3,(H,33,36)(H,34,37)/t23-,28-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373807
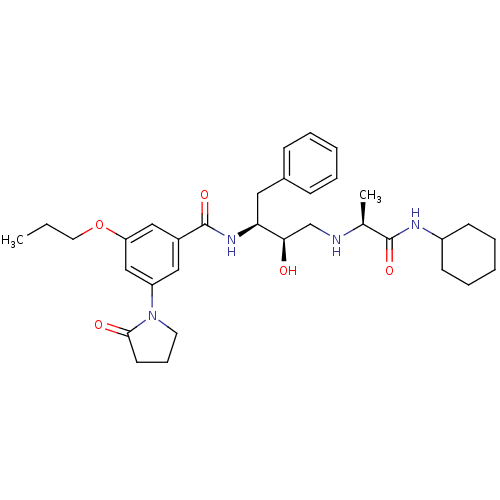
(CHEMBL272714)Show SMILES CCCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O5/c1-3-17-42-28-20-25(19-27(21-28)37-16-10-15-31(37)39)33(41)36-29(18-24-11-6-4-7-12-24)30(38)22-34-23(2)32(40)35-26-13-8-5-9-14-26/h4,6-7,11-12,19-21,23,26,29-30,34,38H,3,5,8-10,13-18,22H2,1-2H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 605 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50373805

(CHEMBL255964)Show SMILES CC(C)CNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C34H49N5O4/c1-23(2)21-36-28-18-26(19-29(20-28)39-16-10-15-32(39)41)34(43)38-30(17-25-11-6-4-7-12-25)31(40)22-35-24(3)33(42)37-27-13-8-5-9-14-27/h4,6-7,11-12,18-20,23-24,27,30-31,35-36,40H,5,8-10,13-17,21-22H2,1-3H3,(H,37,42)(H,38,43)/t24-,30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373804

(CHEMBL257871)Show SMILES CCCc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O4/c1-3-11-25-18-26(21-28(19-25)37-17-10-16-31(37)39)33(41)36-29(20-24-12-6-4-7-13-24)30(38)22-34-23(2)32(40)35-27-14-8-5-9-15-27/h4,6-7,12-13,18-19,21,23,27,29-30,34,38H,3,5,8-11,14-17,20,22H2,1-2H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in SHSY5Y cells assessed as reduction of amyloid beta 42 production |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373811
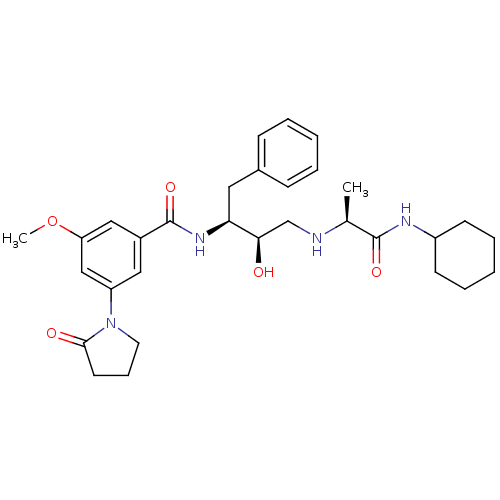
(CHEMBL258277)Show SMILES COc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C31H42N4O5/c1-21(30(38)33-24-12-7-4-8-13-24)32-20-28(36)27(16-22-10-5-3-6-11-22)34-31(39)23-17-25(19-26(18-23)40-2)35-15-9-14-29(35)37/h3,5-6,10-11,17-19,21,24,27-28,32,36H,4,7-9,12-16,20H2,1-2H3,(H,33,38)(H,34,39)/t21-,27-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 745 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50373806

(CHEMBL227053)Show SMILES CC(C)Nc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H47N5O4/c1-22(2)35-27-18-25(19-28(20-27)38-16-10-15-31(38)40)33(42)37-29(17-24-11-6-4-7-12-24)30(39)21-34-23(3)32(41)36-26-13-8-5-9-14-26/h4,6-7,11-12,18-20,22-23,26,29-30,34-35,39H,5,8-10,13-17,21H2,1-3H3,(H,36,41)(H,37,42)/t23-,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373799
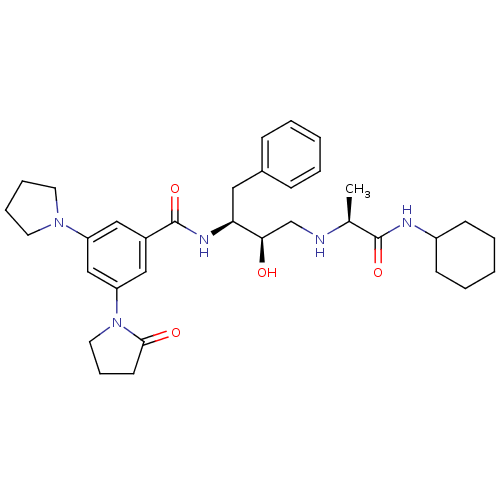
(CHEMBL255192)Show SMILES C[C@H](NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc(cc(c1)N1CCCC1=O)N1CCCC1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C34H47N5O4/c1-24(33(42)36-27-13-6-3-7-14-27)35-23-31(40)30(19-25-11-4-2-5-12-25)37-34(43)26-20-28(38-16-8-9-17-38)22-29(21-26)39-18-10-15-32(39)41/h2,4-5,11-12,20-22,24,27,30-31,35,40H,3,6-10,13-19,23H2,1H3,(H,36,42)(H,37,43)/t24-,30-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373810

(CHEMBL258068)Show SMILES CCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C32H44N4O5/c1-3-41-27-19-24(18-26(20-27)36-16-10-15-30(36)38)32(40)35-28(17-23-11-6-4-7-12-23)29(37)21-33-22(2)31(39)34-25-13-8-5-9-14-25/h4,6-7,11-12,18-20,22,25,28-29,33,37H,3,5,8-10,13-17,21H2,1-2H3,(H,34,39)(H,35,40)/t22-,28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in SHSY5Y cells assessed as reduction of amyloid beta 40 production |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373804

(CHEMBL257871)Show SMILES CCCc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O4/c1-3-11-25-18-26(21-28(19-25)37-17-10-16-31(37)39)33(41)36-29(20-24-12-6-4-7-13-24)30(38)22-34-23(2)32(40)35-27-14-8-5-9-15-27/h4,6-7,12-13,18-19,21,23,27,29-30,34,38H,3,5,8-11,14-17,20,22H2,1-2H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in SHSY5Y cells assessed as reduction of amyloid beta 40 production |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373800

(CHEMBL255977)Show SMILES CC(C)Oc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O5/c1-22(2)42-28-19-25(18-27(20-28)37-16-10-15-31(37)39)33(41)36-29(17-24-11-6-4-7-12-24)30(38)21-34-23(3)32(40)35-26-13-8-5-9-14-26/h4,6-7,11-12,18-20,22-23,26,29-30,34,38H,5,8-10,13-17,21H2,1-3H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in SHSY5Y cells assessed as reduction of amyloid beta 40 production |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373800

(CHEMBL255977)Show SMILES CC(C)Oc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O5/c1-22(2)42-28-19-25(18-27(20-28)37-16-10-15-31(37)39)33(41)36-29(17-24-11-6-4-7-12-24)30(38)21-34-23(3)32(40)35-26-13-8-5-9-14-26/h4,6-7,11-12,18-20,22-23,26,29-30,34,38H,5,8-10,13-17,21H2,1-3H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in SHSY5Y cells assessed as reduction of amyloid beta 42 production |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373807

(CHEMBL272714)Show SMILES CCCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O5/c1-3-17-42-28-20-25(19-27(21-28)37-16-10-15-31(37)39)33(41)36-29(18-24-11-6-4-7-12-24)30(38)22-34-23(2)32(40)35-26-13-8-5-9-14-26/h4,6-7,11-12,19-21,23,26,29-30,34,38H,3,5,8-10,13-18,22H2,1-2H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in SHSY5Y cells assessed as reduction of amyloid beta 42 production |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373810

(CHEMBL258068)Show SMILES CCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C32H44N4O5/c1-3-41-27-19-24(18-26(20-27)36-16-10-15-30(36)38)32(40)35-28(17-23-11-6-4-7-12-23)29(37)21-33-22(2)31(39)34-25-13-8-5-9-14-25/h4,6-7,11-12,18-20,22,25,28-29,33,37H,3,5,8-10,13-17,21H2,1-2H3,(H,34,39)(H,35,40)/t22-,28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in SHSY5Y cells assessed as reduction of amyloid beta 42 production |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373808
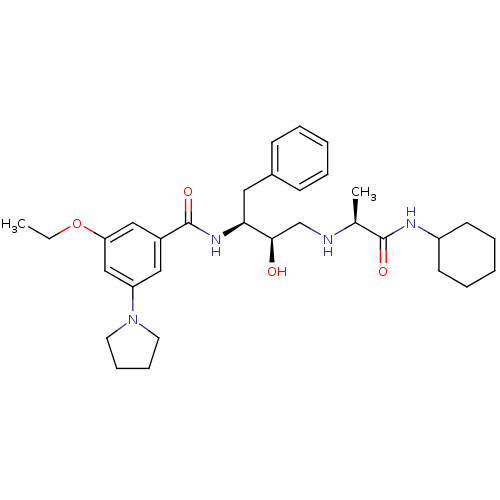
(CHEMBL403533)Show SMILES CCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1 Show InChI InChI=1S/C32H46N4O4/c1-3-40-28-20-25(19-27(21-28)36-16-10-11-17-36)32(39)35-29(18-24-12-6-4-7-13-24)30(37)22-33-23(2)31(38)34-26-14-8-5-9-15-26/h4,6-7,12-13,19-21,23,26,29-30,33,37H,3,5,8-11,14-18,22H2,1-2H3,(H,34,38)(H,35,39)/t23-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50373804

(CHEMBL257871)Show SMILES CCCc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O4/c1-3-11-25-18-26(21-28(19-25)37-17-10-16-31(37)39)33(41)36-29(20-24-12-6-4-7-13-24)30(38)22-34-23(2)32(40)35-27-14-8-5-9-15-27/h4,6-7,12-13,18-19,21,23,27,29-30,34,38H,3,5,8-11,14-17,20,22H2,1-2H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373807

(CHEMBL272714)Show SMILES CCCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O5/c1-3-17-42-28-20-25(19-27(21-28)37-16-10-15-31(37)39)33(41)36-29(18-24-11-6-4-7-12-24)30(38)22-34-23(2)32(40)35-26-13-8-5-9-14-26/h4,6-7,11-12,19-21,23,26,29-30,34,38H,3,5,8-10,13-18,22H2,1-2H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in SHSY5Y cells assessed as reduction of amyloid beta 40 production |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373812

(CHEMBL402305)Show SMILES CCCCCS(=O)(=O)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C31H45N3O5S/c1-3-4-11-19-40(38,39)27-18-12-15-25(21-27)31(37)34-28(20-24-13-7-5-8-14-24)29(35)22-32-23(2)30(36)33-26-16-9-6-10-17-26/h5,7-8,12-15,18,21,23,26,28-29,32,35H,3-4,6,9-11,16-17,19-20,22H2,1-2H3,(H,33,36)(H,34,37)/t23-,28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50231682

(CHEMBL251862 | N-((2S,3R)-4-((S)-1-(cyclohexylamin...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C32H45N5O4/c1-3-33-26-18-24(19-27(20-26)37-16-10-15-30(37)39)32(41)36-28(17-23-11-6-4-7-12-23)29(38)21-34-22(2)31(40)35-25-13-8-5-9-14-25/h4,6-7,11-12,18-20,22,25,28-29,33-34,38H,3,5,8-10,13-17,21H2,1-2H3,(H,35,40)(H,36,41)/t22-,28-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50373813
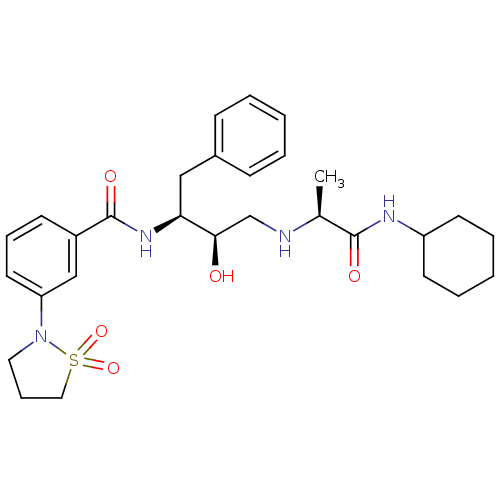
(CHEMBL271437)Show SMILES C[C@H](NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(c1)N1CCCS1(=O)=O)C(=O)NC1CCCCC1 Show InChI InChI=1S/C29H40N4O5S/c1-21(28(35)31-24-13-6-3-7-14-24)30-20-27(34)26(18-22-10-4-2-5-11-22)32-29(36)23-12-8-15-25(19-23)33-16-9-17-39(33,37)38/h2,4-5,8,10-12,15,19,21,24,26-27,30,34H,3,6-7,9,13-14,16-18,20H2,1H3,(H,31,35)(H,32,36)/t21-,26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50231682

(CHEMBL251862 | N-((2S,3R)-4-((S)-1-(cyclohexylamin...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C32H45N5O4/c1-3-33-26-18-24(19-27(20-26)37-16-10-15-30(37)39)32(41)36-28(17-23-11-6-4-7-12-23)29(38)21-34-22(2)31(40)35-25-13-8-5-9-14-25/h4,6-7,11-12,18-20,22,25,28-29,33-34,38H,3,5,8-10,13-17,21H2,1-2H3,(H,35,40)(H,36,41)/t22-,28-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50373805

(CHEMBL255964)Show SMILES CC(C)CNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C34H49N5O4/c1-23(2)21-36-28-18-26(19-29(20-28)39-16-10-15-32(39)41)34(43)38-30(17-25-11-6-4-7-12-25)31(40)22-35-24(3)33(42)37-27-13-8-5-9-14-27/h4,6-7,11-12,18-20,23-24,27,30-31,35-36,40H,5,8-10,13-17,21-22H2,1-3H3,(H,37,42)(H,38,43)/t24-,30-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50373812

(CHEMBL402305)Show SMILES CCCCCS(=O)(=O)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C31H45N3O5S/c1-3-4-11-19-40(38,39)27-18-12-15-25(21-27)31(37)34-28(20-24-13-7-5-8-14-24)29(35)22-32-23(2)30(36)33-26-16-9-6-10-17-26/h5,7-8,12-15,18,21,23,26,28-29,32,35H,3-4,6,9-11,16-17,19-20,22H2,1-2H3,(H,33,36)(H,34,37)/t23-,28-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50373804

(CHEMBL257871)Show SMILES CCCc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O4/c1-3-11-25-18-26(21-28(19-25)37-17-10-16-31(37)39)33(41)36-29(20-24-12-6-4-7-13-24)30(38)22-34-23(2)32(40)35-27-14-8-5-9-15-27/h4,6-7,12-13,18-19,21,23,27,29-30,34,38H,3,5,8-11,14-17,20,22H2,1-2H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50373800

(CHEMBL255977)Show SMILES CC(C)Oc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O5/c1-22(2)42-28-19-25(18-27(20-28)37-16-10-15-31(37)39)33(41)36-29(17-24-11-6-4-7-12-24)30(38)21-34-23(3)32(40)35-26-13-8-5-9-14-26/h4,6-7,11-12,18-20,22-23,26,29-30,34,38H,5,8-10,13-17,21H2,1-3H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50373810

(CHEMBL258068)Show SMILES CCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C32H44N4O5/c1-3-41-27-19-24(18-26(20-27)36-16-10-15-30(36)38)32(40)35-28(17-23-11-6-4-7-12-23)29(37)21-33-22(2)31(39)34-25-13-8-5-9-14-25/h4,6-7,11-12,18-20,22,25,28-29,33,37H,3,5,8-10,13-17,21H2,1-2H3,(H,34,39)(H,35,40)/t22-,28-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50322878
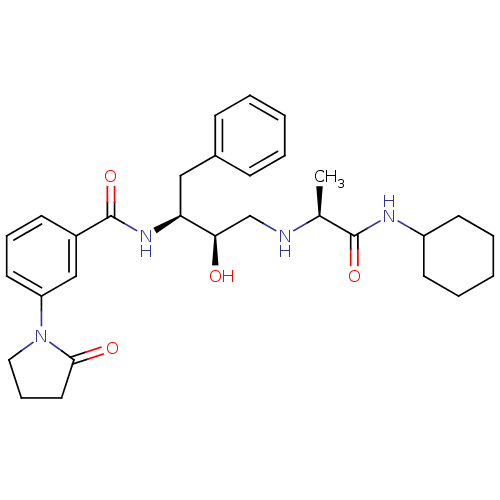
(CHEMBL271230 | N-((2S,3R)-4-((S)-1-(cyclohexylamin...)Show SMILES C[C@H](NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(c1)N1CCCC1=O)C(=O)NC1CCCCC1 Show InChI InChI=1S/C30H40N4O4/c1-21(29(37)32-24-13-6-3-7-14-24)31-20-27(35)26(18-22-10-4-2-5-11-22)33-30(38)23-12-8-15-25(19-23)34-17-9-16-28(34)36/h2,4-5,8,10-12,15,19,21,24,26-27,31,35H,3,6-7,9,13-14,16-18,20H2,1H3,(H,32,37)(H,33,38)/t21-,26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50231691
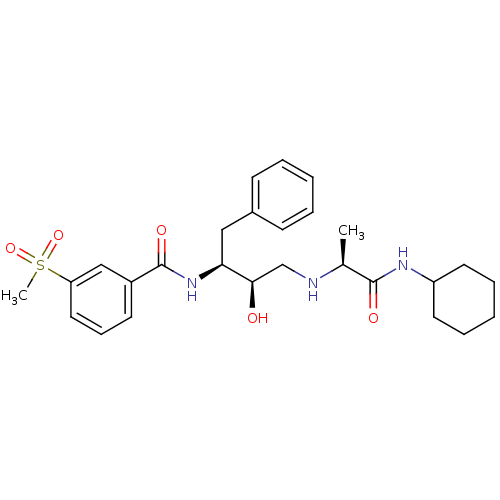
(CHEMBL402120 | N-((2S,3R)-4-((S)-1-(cyclohexylamin...)Show SMILES C[C@H](NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(c1)S(C)(=O)=O)C(=O)NC1CCCCC1 Show InChI InChI=1S/C27H37N3O5S/c1-19(26(32)29-22-13-7-4-8-14-22)28-18-25(31)24(16-20-10-5-3-6-11-20)30-27(33)21-12-9-15-23(17-21)36(2,34)35/h3,5-6,9-12,15,17,19,22,24-25,28,31H,4,7-8,13-14,16,18H2,1-2H3,(H,29,32)(H,30,33)/t19-,24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50373806

(CHEMBL227053)Show SMILES CC(C)Nc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H47N5O4/c1-22(2)35-27-18-25(19-28(20-27)38-16-10-15-31(38)40)33(42)37-29(17-24-11-6-4-7-12-24)30(39)21-34-23(3)32(41)36-26-13-8-5-9-14-26/h4,6-7,11-12,18-20,22-23,26,29-30,34-35,39H,5,8-10,13-17,21H2,1-3H3,(H,36,41)(H,37,42)/t23-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50373808

(CHEMBL403533)Show SMILES CCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1 Show InChI InChI=1S/C32H46N4O4/c1-3-40-28-20-25(19-27(21-28)36-16-10-11-17-36)32(39)35-29(18-24-12-6-4-7-13-24)30(37)22-33-23(2)31(38)34-26-14-8-5-9-15-26/h4,6-7,12-13,19-21,23,26,29-30,33,37H,3,5,8-11,14-18,22H2,1-2H3,(H,34,38)(H,35,39)/t23-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50231691

(CHEMBL402120 | N-((2S,3R)-4-((S)-1-(cyclohexylamin...)Show SMILES C[C@H](NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(c1)S(C)(=O)=O)C(=O)NC1CCCCC1 Show InChI InChI=1S/C27H37N3O5S/c1-19(26(32)29-22-13-7-4-8-14-22)28-18-25(31)24(16-20-10-5-3-6-11-20)30-27(33)21-12-9-15-23(17-21)36(2,34)35/h3,5-6,9-12,15,17,19,22,24-25,28,31H,4,7-8,13-14,16,18H2,1-2H3,(H,29,32)(H,30,33)/t19-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50373810

(CHEMBL258068)Show SMILES CCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C32H44N4O5/c1-3-41-27-19-24(18-26(20-27)36-16-10-15-30(36)38)32(40)35-28(17-23-11-6-4-7-12-23)29(37)21-33-22(2)31(39)34-25-13-8-5-9-14-25/h4,6-7,11-12,18-20,22,25,28-29,33,37H,3,5,8-10,13-17,21H2,1-2H3,(H,34,39)(H,35,40)/t22-,28-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50373807

(CHEMBL272714)Show SMILES CCCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O5/c1-3-17-42-28-20-25(19-27(21-28)37-16-10-15-31(37)39)33(41)36-29(18-24-11-6-4-7-12-24)30(38)22-34-23(2)32(40)35-26-13-8-5-9-14-26/h4,6-7,11-12,19-21,23,26,29-30,34,38H,3,5,8-10,13-18,22H2,1-2H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50373799

(CHEMBL255192)Show SMILES C[C@H](NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc(cc(c1)N1CCCC1=O)N1CCCC1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C34H47N5O4/c1-24(33(42)36-27-13-6-3-7-14-27)35-23-31(40)30(19-25-11-4-2-5-12-25)37-34(43)26-20-28(38-16-8-9-17-38)22-29(21-26)39-18-10-15-32(39)41/h2,4-5,11-12,20-22,24,27,30-31,35,40H,3,6-10,13-19,23H2,1H3,(H,36,42)(H,37,43)/t24-,30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50373799

(CHEMBL255192)Show SMILES C[C@H](NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc(cc(c1)N1CCCC1=O)N1CCCC1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C34H47N5O4/c1-24(33(42)36-27-13-6-3-7-14-27)35-23-31(40)30(19-25-11-4-2-5-12-25)37-34(43)26-20-28(38-16-8-9-17-38)22-29(21-26)39-18-10-15-32(39)41/h2,4-5,11-12,20-22,24,27,30-31,35,40H,3,6-10,13-19,23H2,1H3,(H,36,42)(H,37,43)/t24-,30-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50373813

(CHEMBL271437)Show SMILES C[C@H](NC[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(c1)N1CCCS1(=O)=O)C(=O)NC1CCCCC1 Show InChI InChI=1S/C29H40N4O5S/c1-21(28(35)31-24-13-6-3-7-14-24)30-20-27(34)26(18-22-10-4-2-5-11-22)32-29(36)23-12-8-15-25(19-23)33-16-9-17-39(33,37)38/h2,4-5,8,10-12,15,19,21,24,26-27,30,34H,3,6-7,9,13-14,16-18,20H2,1H3,(H,31,35)(H,32,36)/t21-,26-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50373800

(CHEMBL255977)Show SMILES CC(C)Oc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1=O Show InChI InChI=1S/C33H46N4O5/c1-22(2)42-28-19-25(18-27(20-28)37-16-10-15-31(37)39)33(41)36-29(17-24-11-6-4-7-12-24)30(38)21-34-23(3)32(40)35-26-13-8-5-9-14-26/h4,6-7,11-12,18-20,22-23,26,29-30,34,38H,5,8-10,13-17,21H2,1-3H3,(H,35,40)(H,36,41)/t23-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50373808

(CHEMBL403533)Show SMILES CCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N1CCCC1 Show InChI InChI=1S/C32H46N4O4/c1-3-40-28-20-25(19-27(21-28)36-16-10-11-17-36)32(39)35-29(18-24-12-6-4-7-13-24)30(37)22-33-23(2)31(38)34-26-14-8-5-9-15-26/h4,6-7,12-13,19-21,23,26,29-30,33,37H,3,5,8-11,14-18,22H2,1-2H3,(H,34,38)(H,35,39)/t23-,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 18: 1011-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.017
BindingDB Entry DOI: 10.7270/Q2PG1SK5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data