

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537670 (CHEMBL4641380) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Tested for inhibition of cGMP-dependent protein kinase from bovine lung | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537669 (CHEMBL4643608) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537671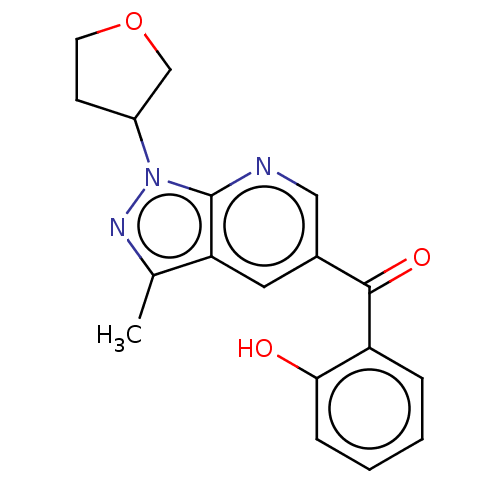 (CHEMBL4633843) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Tested for inhibition of cGMP-dependent protein kinase from bovine lung | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537648 (CHEMBL4640596) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537661 (CHEMBL4648946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537646 (CHEMBL512270) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537649 (CHEMBL1723285) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor autophosphorylation in A431 human epidermoid carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537665 (CHEMBL4648066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537662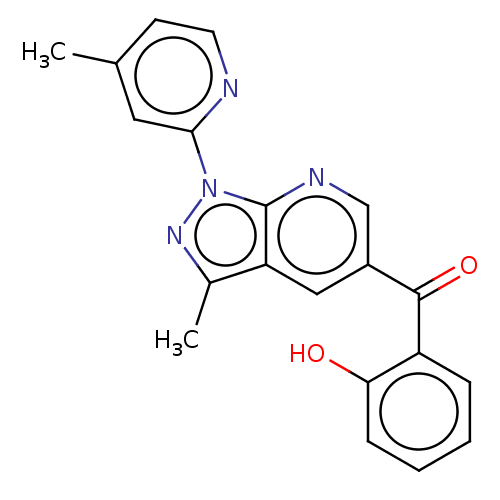 (CHEMBL4647748) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537643 (CHEMBL1506565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537629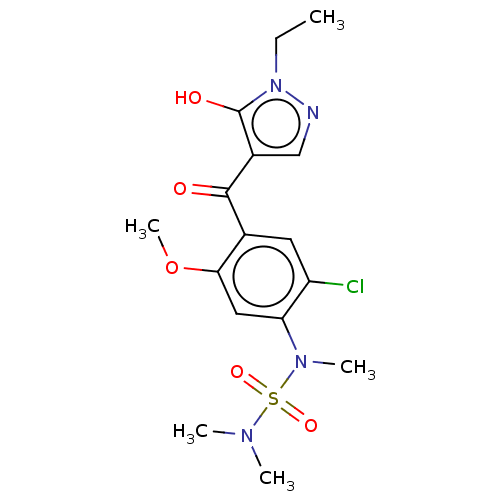 (CHEMBL4635339) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537628 (CHEMBL4643853) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537628 (CHEMBL4643853) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 66.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Tested for inhibition of cGMP-dependent protein kinase from bovine lung | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537659 (CHEMBL4646298) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537647 (CHEMBL1403506) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537667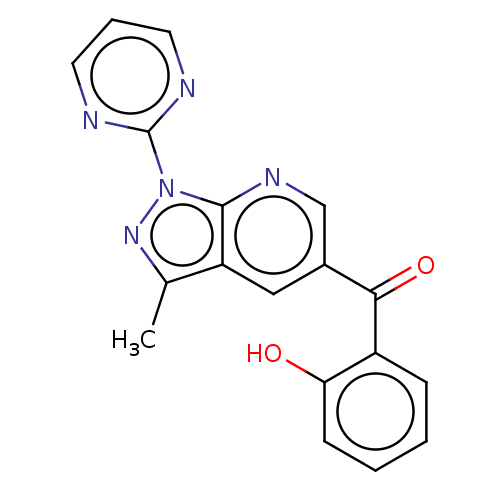 (CHEMBL4639207) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537668 (CHEMBL4647815) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537652 (CHEMBL4640293) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537639 (CHEMBL4639296) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537641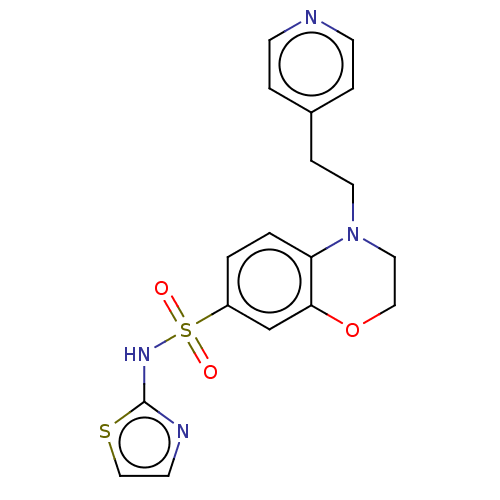 (CHEMBL4647303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 575 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537626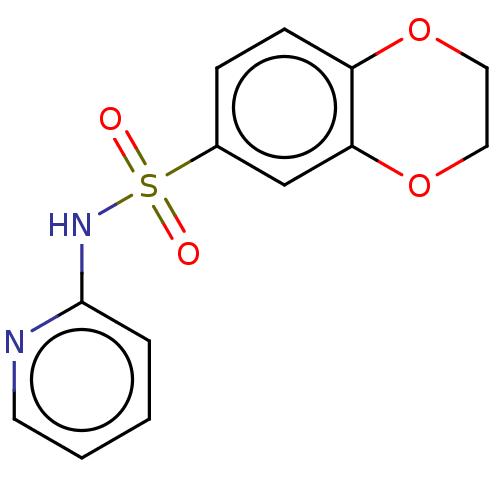 (CHEMBL4645869) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 603 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor autophosphorylation in A431 human epidermoid carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537664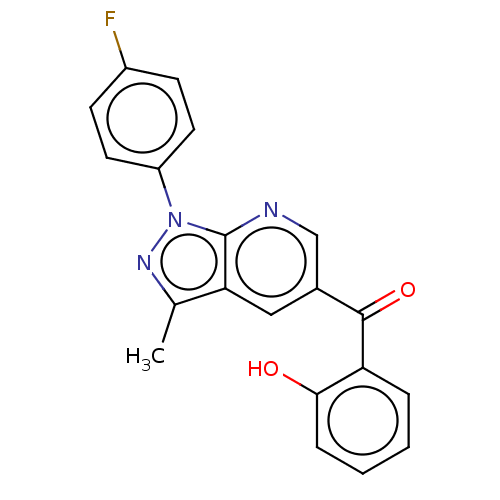 (CHEMBL4637016) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537653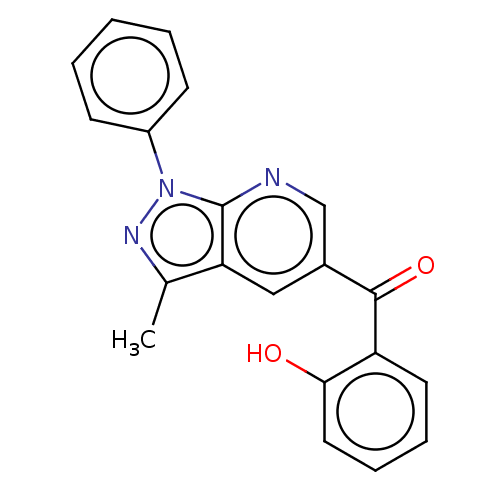 (CHEMBL4643582) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537645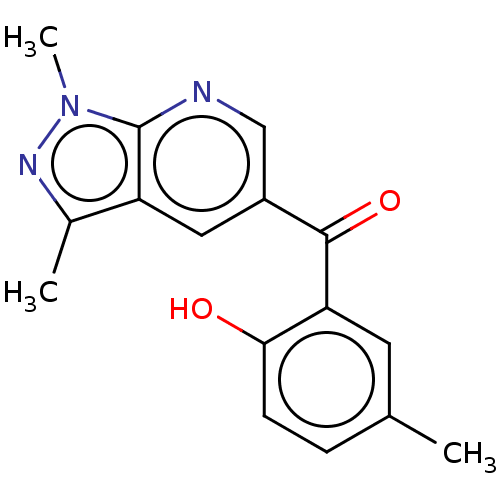 (CHEMBL467988) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537637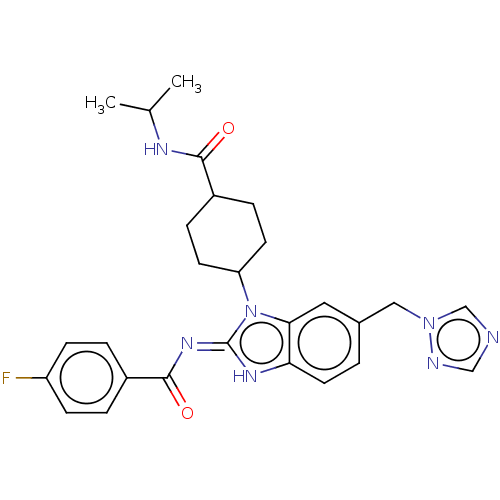 (CHEMBL4644624) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor autophosphorylation in A431 human epidermoid carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537655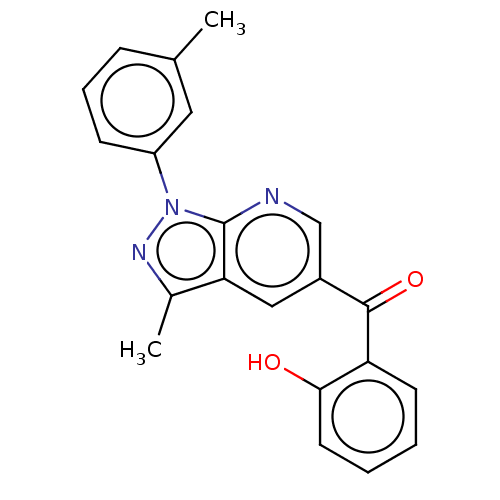 (CHEMBL4649728) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537660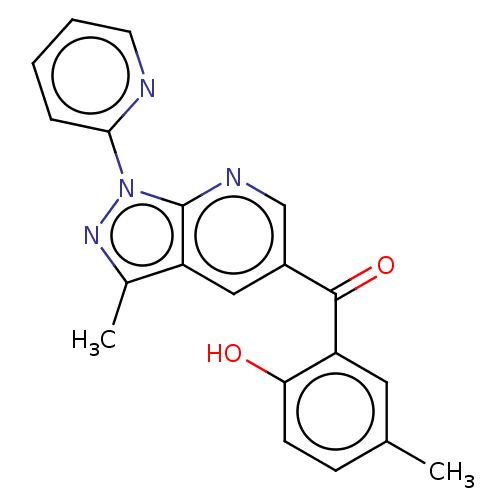 (CHEMBL4634222) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM154387 (US9012443, 427) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM154206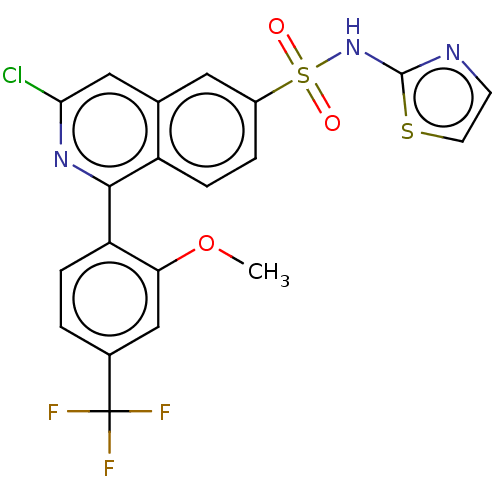 (US9012443, 219) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537644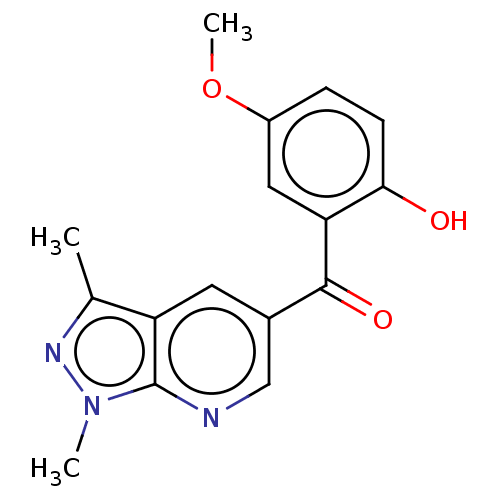 (CHEMBL1582054) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537638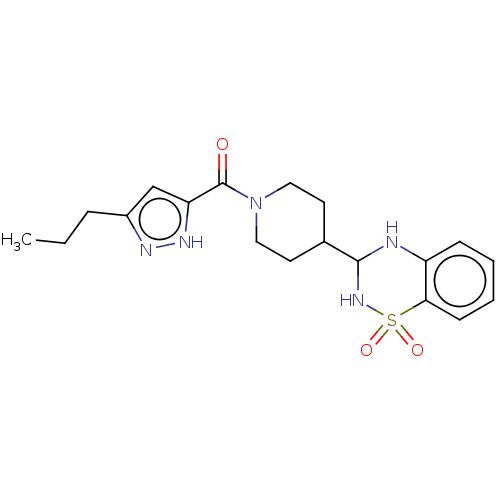 (CHEMBL4648561) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537635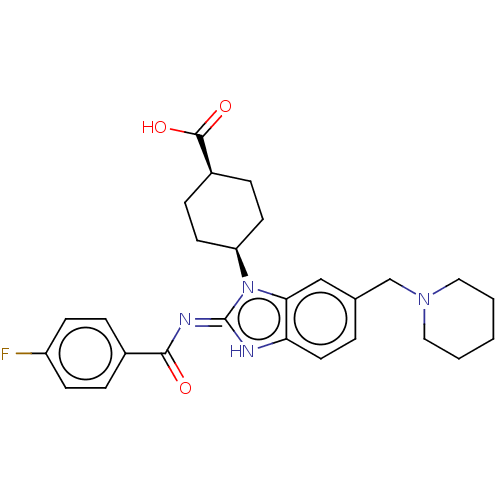 (CHEMBL4634771) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537633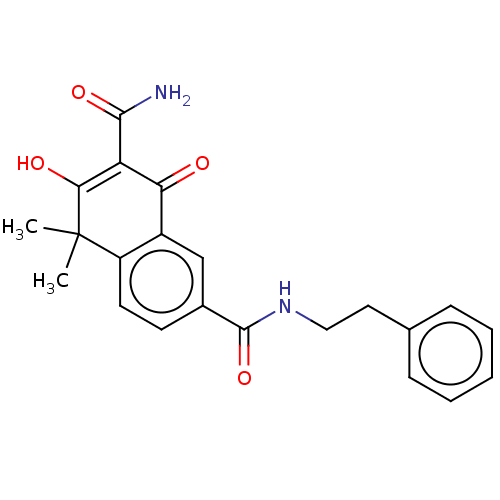 (CHEMBL4635573) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor autophosphorylation in A431 human epidermoid carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537630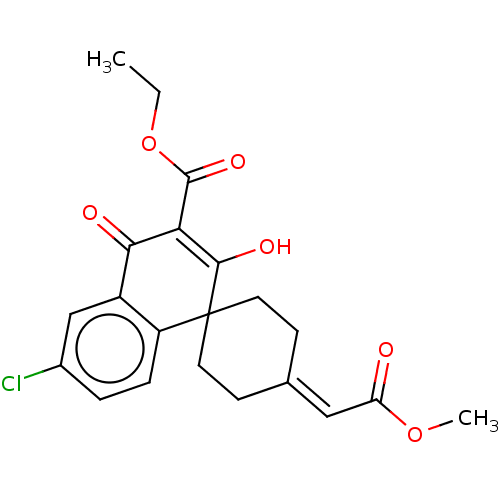 (CHEMBL4633966) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537642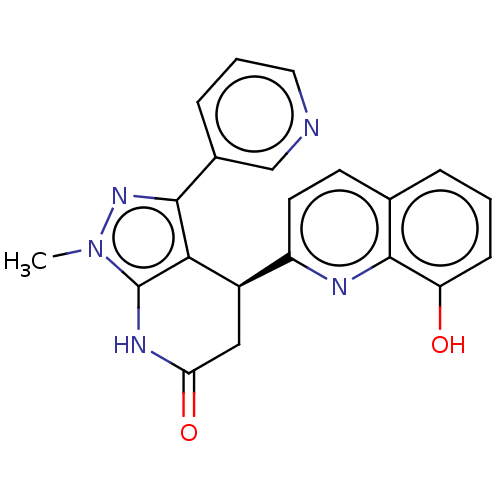 (CHEMBL4638594) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537632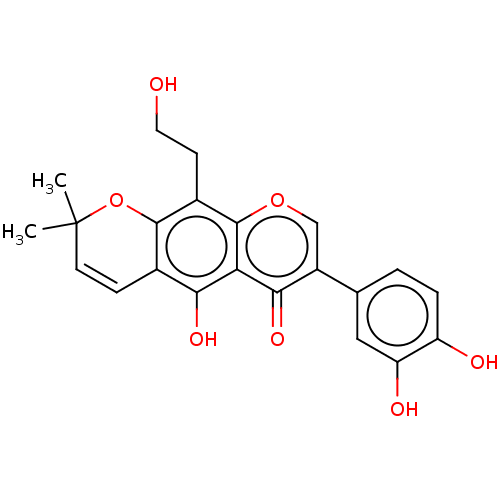 (CHEMBL4594142) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537627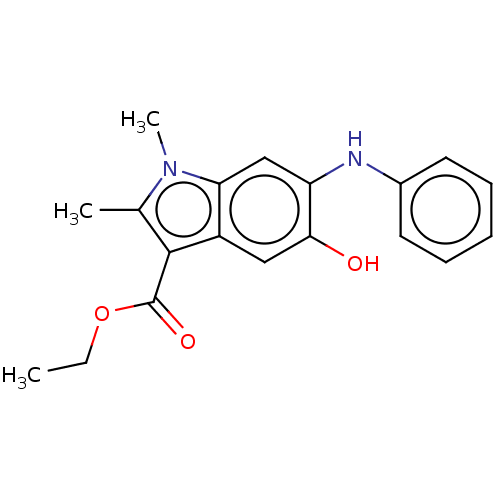 (CHEMBL4641013) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537634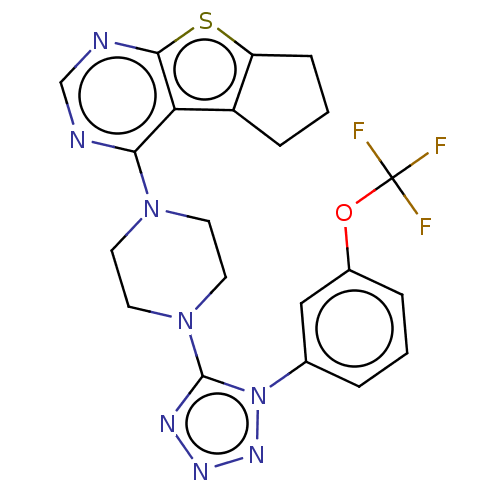 (CHEMBL4639502) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537625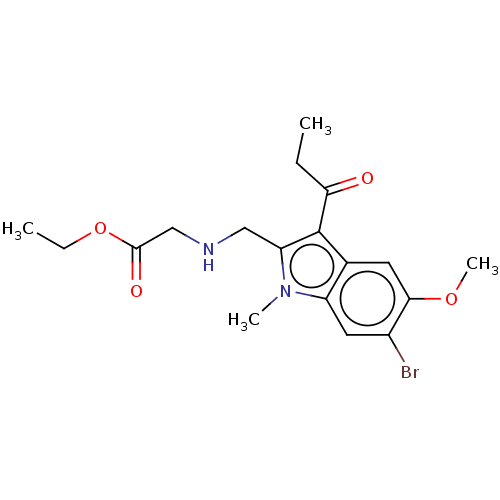 (CHEMBL4648044) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537624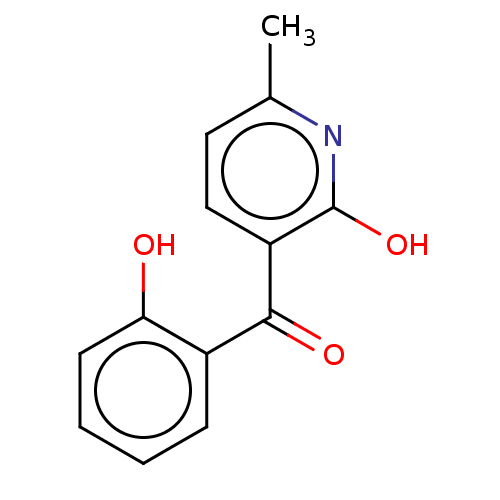 (CHEMBL4635045) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537640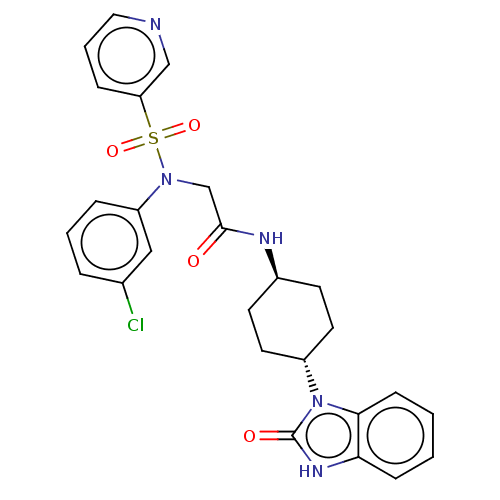 (CHEMBL4637484) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537631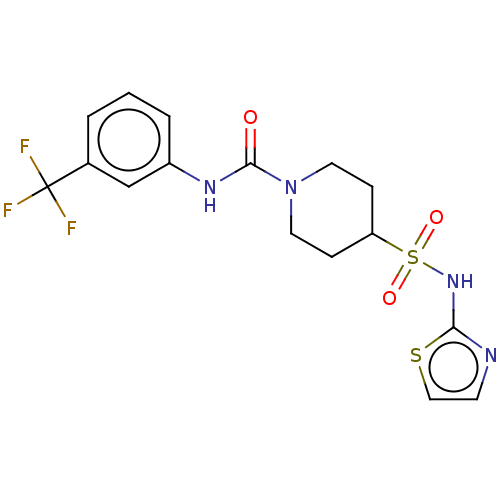 (CHEMBL4636699) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537636 (CHEMBL4648024) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of Epidermal growth factor receptor autophosphorylation in A431 human epidermoid carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537650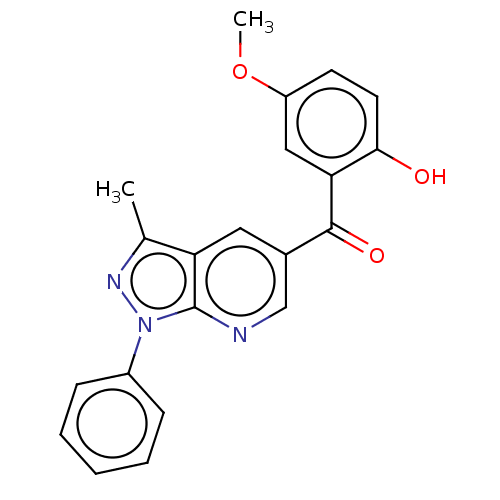 (CHEMBL4635913) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537656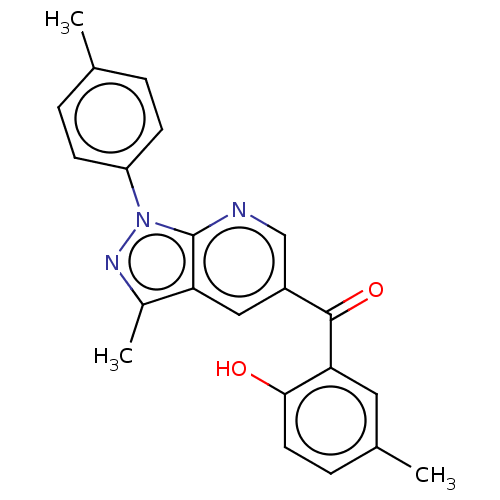 (CHEMBL4635842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537658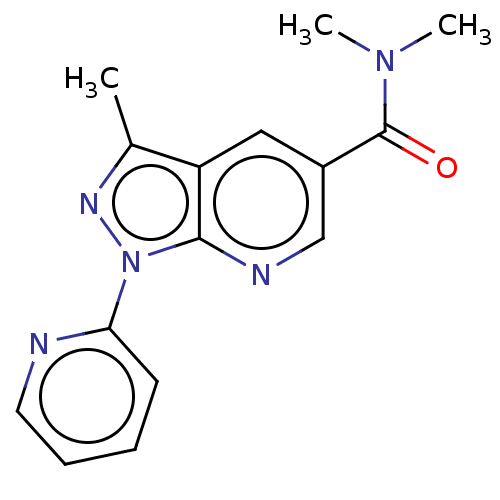 (CHEMBL4634336) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537654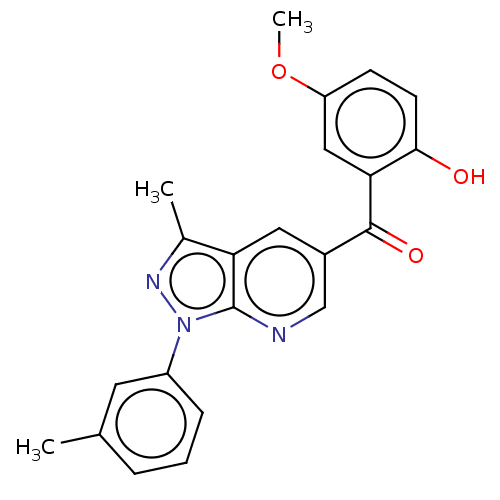 (CHEMBL4643587) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537663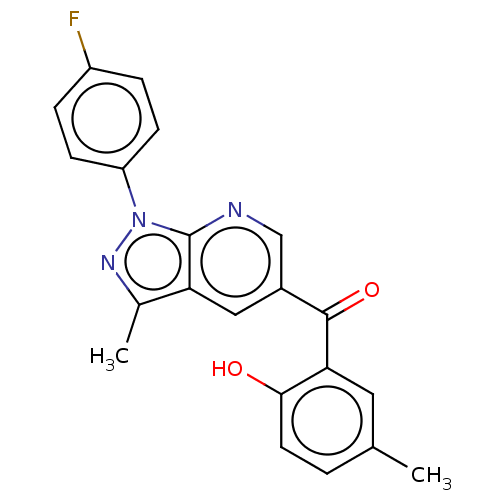 (CHEMBL4636826) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537666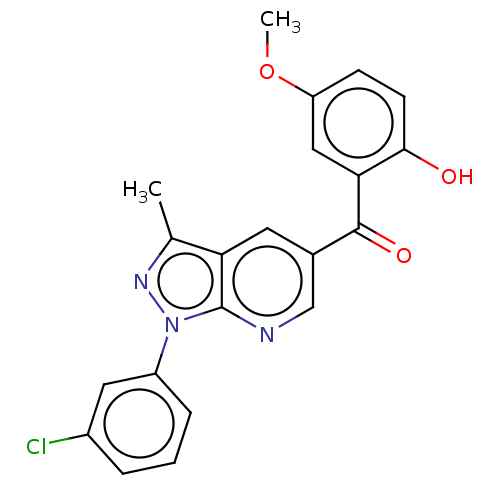 (CHEMBL4633879) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sepiapterin reductase (Homo sapiens (Human)) | BDBM50537657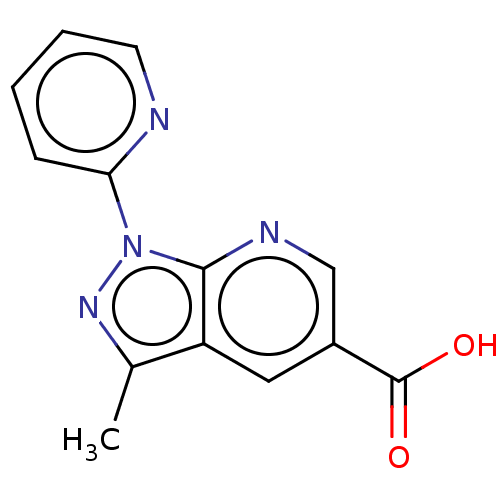 (CHEMBL4636874) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at CB1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126793 BindingDB Entry DOI: 10.7270/Q2Z89G99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |