 Found 59 hits Enz. Inhib. hit(s) with all data for entry = 50047288
Found 59 hits Enz. Inhib. hit(s) with all data for entry = 50047288 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50157880
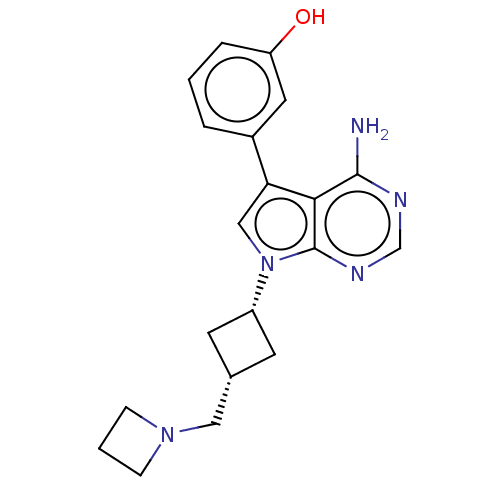
(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Ret (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157878
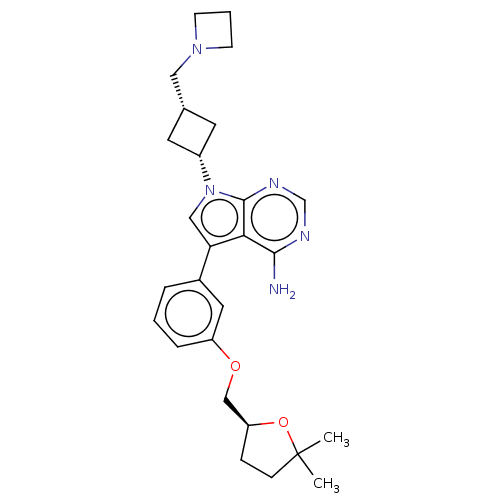
(CHEMBL3786567)Show SMILES CC1(C)CC[C@@H](COc2cccc(c2)-c2cn([C@@H]3C[C@H](CN4CCC4)C3)c3ncnc(N)c23)O1 |r,wU:5.5,wD:17.17,19.20,(-1.8,11.77,;-1.51,10.57,;-2.39,9.7,;-.3,11.52,;.98,10.66,;.54,9.2,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.56,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,;-.98,9.12,)| Show InChI InChI=1S/C27H35N5O2/c1-27(2)8-7-22(34-27)16-33-21-6-3-5-19(13-21)23-15-32(26-24(23)25(28)29-17-30-26)20-11-18(12-20)14-31-9-4-10-31/h3,5-6,13,15,17-18,20,22H,4,7-12,14,16H2,1-2H3,(H2,28,29,30)/t18-,20+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157888
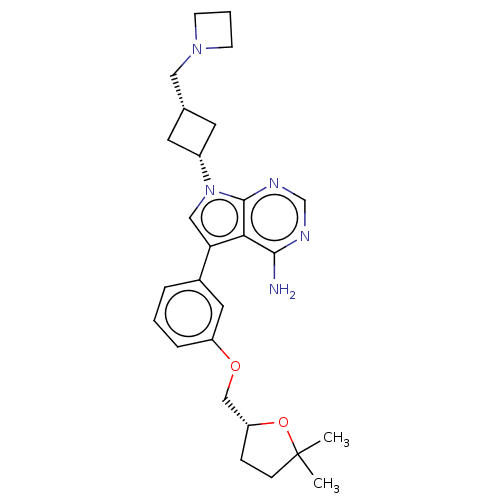
(CHEMBL3785273)Show SMILES CC1(C)CC[C@H](COc2cccc(c2)-c2cn([C@@H]3C[C@H](CN4CCC4)C3)c3ncnc(N)c23)O1 |r,wD:17.17,19.20,5.5,(-1.8,11.77,;-1.51,10.57,;-2.39,9.7,;-.3,11.52,;.98,10.66,;.54,9.2,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.56,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,;-.98,9.12,)| Show InChI InChI=1S/C27H35N5O2/c1-27(2)8-7-22(34-27)16-33-21-6-3-5-19(13-21)23-15-32(26-24(23)25(28)29-17-30-26)20-11-18(12-20)14-31-9-4-10-31/h3,5-6,13,15,17-18,20,22H,4,7-12,14,16H2,1-2H3,(H2,28,29,30)/t18-,20+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157879
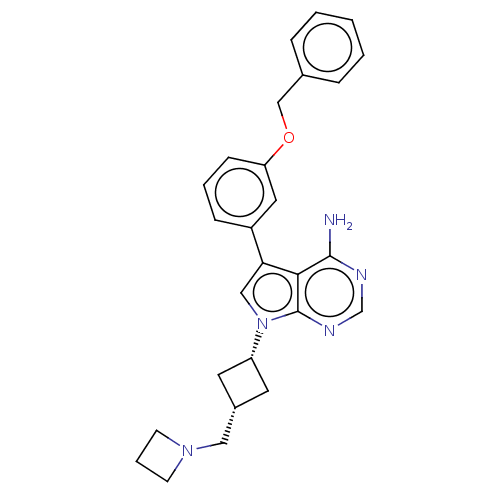
(CHEMBL1614712)Show SMILES [H][C@]1(CN2CCC2)C[C@]([H])(C1)n1cc(-c2cccc(OCc3ccccc3)c2)c2c(N)ncnc12 |wD:8.9,1.0,(6.47,-15.11,;5.71,-13.78,;4.93,-15.11,;5.69,-16.44,;5.68,-17.98,;7.22,-17.99,;7.23,-16.45,;5.31,-12.3,;6.81,-11.9,;6.02,-10.55,;7.2,-13.39,;7.59,-10.56,;6.61,-9.37,;7.45,-8.07,;7.04,-6.58,;5.56,-6.19,;5.15,-4.71,;6.24,-3.6,;7.73,-3.99,;8.81,-2.89,;10.15,-3.66,;11.48,-2.89,;12.81,-3.66,;14.12,-2.9,;14.13,-1.36,;12.8,-.59,;11.48,-1.36,;8.13,-5.47,;8.94,-8.47,;10.35,-7.7,;10.34,-6.15,;11.69,-8.46,;11.69,-10.01,;10.35,-10.79,;9.02,-10.01,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(21-8-4-9-23(14-21)33-17-19-6-2-1-3-7-19)16-32(27(25)30-18-29-26)22-12-20(13-22)15-31-10-5-11-31/h1-4,6-9,14,16,18,20,22H,5,10-13,15,17H2,(H2,28,29,30)/t20-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157894
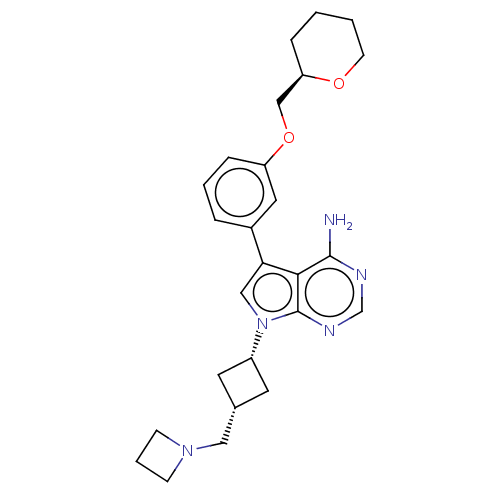
(CHEMBL3786383)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@H]4CCCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wU:16.15,wD:24.27,26.30,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,8.98,;-1.93,10.19,;-1.36,11.62,;.17,11.84,;1.12,10.63,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C26H33N5O2/c27-25-24-23(19-5-3-7-21(13-19)33-16-22-6-1-2-10-32-22)15-31(26(24)29-17-28-25)20-11-18(12-20)14-30-8-4-9-30/h3,5,7,13,15,17-18,20,22H,1-2,4,6,8-12,14,16H2,(H2,27,28,29)/t18-,20+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157881
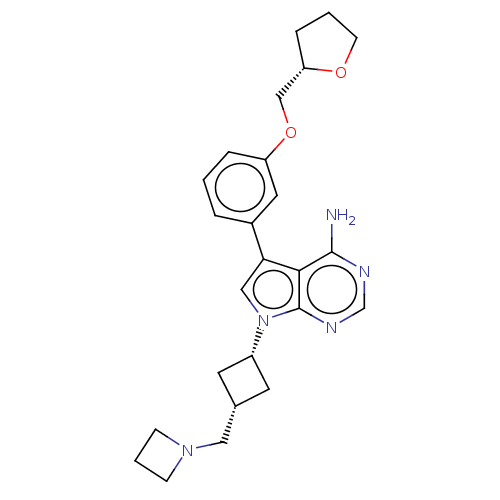
(CHEMBL3787614)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@@H]4CCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:23.26,16.15,25.29,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C25H31N5O2/c26-24-23-22(18-4-1-5-20(12-18)32-15-21-6-2-9-31-21)14-30(25(23)28-16-27-24)19-10-17(11-19)13-29-7-3-8-29/h1,4-5,12,14,16-17,19,21H,2-3,6-11,13,15H2,(H2,26,27,28)/t17-,19+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50157878

(CHEMBL3786567)Show SMILES CC1(C)CC[C@@H](COc2cccc(c2)-c2cn([C@@H]3C[C@H](CN4CCC4)C3)c3ncnc(N)c23)O1 |r,wU:5.5,wD:17.17,19.20,(-1.8,11.77,;-1.51,10.57,;-2.39,9.7,;-.3,11.52,;.98,10.66,;.54,9.2,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.56,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,;-.98,9.12,)| Show InChI InChI=1S/C27H35N5O2/c1-27(2)8-7-22(34-27)16-33-21-6-3-5-19(13-21)23-15-32(26-24(23)25(28)29-17-30-26)20-11-18(12-20)14-31-9-4-10-31/h3,5-6,13,15,17-18,20,22H,4,7-12,14,16H2,1-2H3,(H2,28,29,30)/t18-,20+,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Ins receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157887
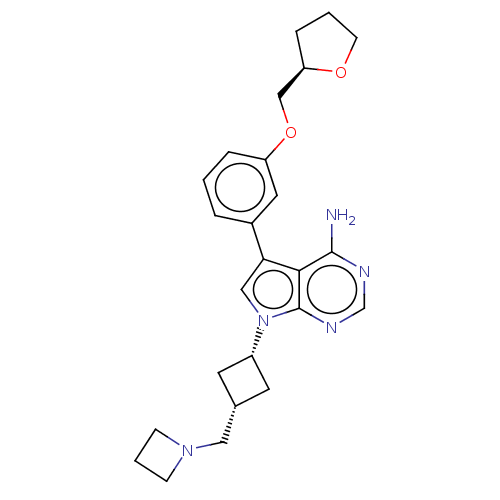
(CHEMBL3786793)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@H]4CCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wU:16.15,wD:23.26,25.29,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C25H31N5O2/c26-24-23-22(18-4-1-5-20(12-18)32-15-21-6-2-9-31-21)14-30(25(23)28-16-27-24)19-10-17(11-19)13-29-7-3-8-29/h1,4-5,12,14,16-17,19,21H,2-3,6-11,13,15H2,(H2,26,27,28)/t17-,19+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157895
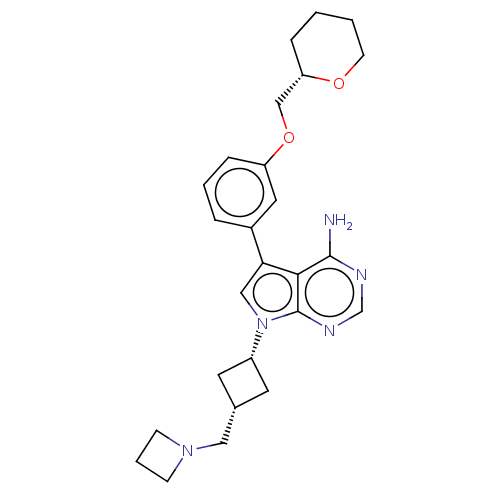
(CHEMBL3787668)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@@H]4CCCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:24.27,26.30,16.15,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,8.98,;-1.93,10.19,;-1.36,11.62,;.17,11.84,;1.12,10.63,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C26H33N5O2/c27-25-24-23(19-5-3-7-21(13-19)33-16-22-6-1-2-10-32-22)15-31(26(24)29-17-28-25)20-11-18(12-20)14-30-8-4-9-30/h3,5,7,13,15,17-18,20,22H,1-2,4,6,8-12,14,16H2,(H2,27,28,29)/t18-,20+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50157879

(CHEMBL1614712)Show SMILES [H][C@]1(CN2CCC2)C[C@]([H])(C1)n1cc(-c2cccc(OCc3ccccc3)c2)c2c(N)ncnc12 |wD:8.9,1.0,(6.47,-15.11,;5.71,-13.78,;4.93,-15.11,;5.69,-16.44,;5.68,-17.98,;7.22,-17.99,;7.23,-16.45,;5.31,-12.3,;6.81,-11.9,;6.02,-10.55,;7.2,-13.39,;7.59,-10.56,;6.61,-9.37,;7.45,-8.07,;7.04,-6.58,;5.56,-6.19,;5.15,-4.71,;6.24,-3.6,;7.73,-3.99,;8.81,-2.89,;10.15,-3.66,;11.48,-2.89,;12.81,-3.66,;14.12,-2.9,;14.13,-1.36,;12.8,-.59,;11.48,-1.36,;8.13,-5.47,;8.94,-8.47,;10.35,-7.7,;10.34,-6.15,;11.69,-8.46,;11.69,-10.01,;10.35,-10.79,;9.02,-10.01,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(21-8-4-9-23(14-21)33-17-19-6-2-1-3-7-19)16-32(27(25)30-18-29-26)22-12-20(13-22)15-31-10-5-11-31/h1-4,6-9,14,16,18,20,22H,5,10-13,15,17H2,(H2,28,29,30)/t20-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157900
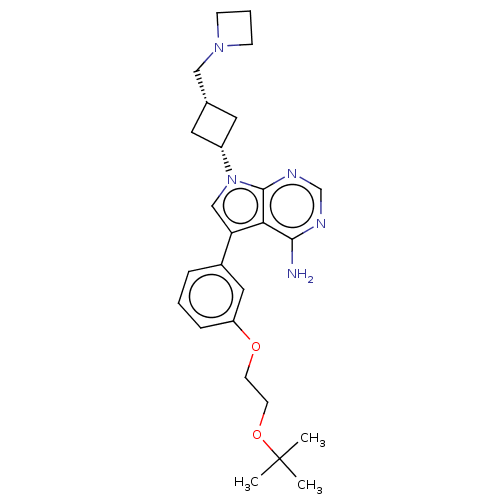
(CHEMBL3786598)Show SMILES CC(C)(C)OCCOc1cccc(c1)-c1cn([C@@H]2C[C@H](CN3CCC3)C2)c2ncnc(N)c12 |r,wD:17.17,19.20,(.63,12.98,;.17,11.84,;-1.05,11.67,;-.59,12.81,;1.12,10.63,;.54,9.2,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.56,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,)| Show InChI InChI=1S/C26H35N5O2/c1-26(2,3)33-11-10-32-21-7-4-6-19(14-21)22-16-31(25-23(22)24(27)28-17-29-25)20-12-18(13-20)15-30-8-5-9-30/h4,6-7,14,16-18,20H,5,8-13,15H2,1-3H3,(H2,27,28,29)/t18-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-beta (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157899
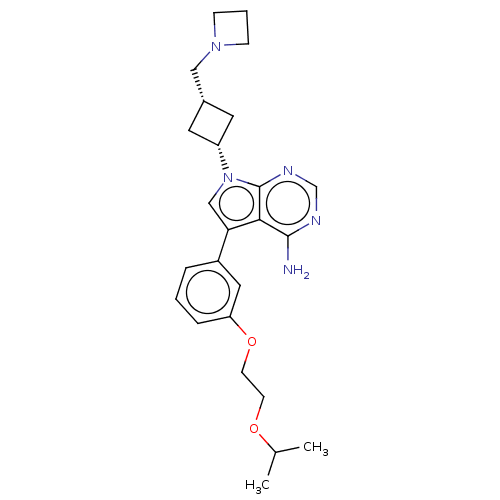
(CHEMBL3786472)Show SMILES CC(C)OCCOc1cccc(c1)-c1cn([C@@H]2C[C@H](CN3CCC3)C2)c2ncnc(N)c12 |r,wD:16.16,18.19,(.63,12.98,;.17,11.84,;-1.05,11.67,;1.12,10.63,;.54,9.2,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.56,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,)| Show InChI InChI=1S/C25H33N5O2/c1-17(2)31-9-10-32-21-6-3-5-19(13-21)22-15-30(25-23(22)24(26)27-16-28-25)20-11-18(12-20)14-29-7-4-8-29/h3,5-6,13,15-18,20H,4,7-12,14H2,1-2H3,(H2,26,27,28)/t18-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Jak1 (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157898
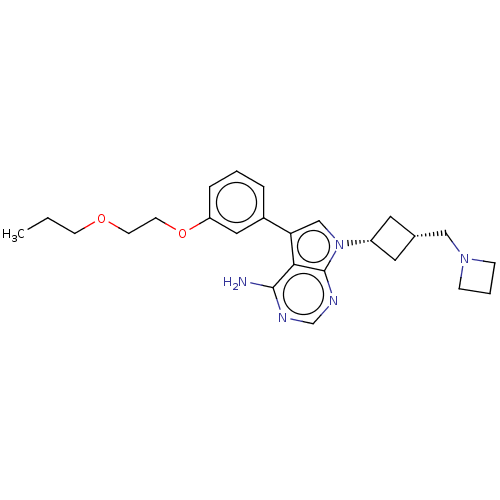
(CHEMBL3785927)Show SMILES CCCOCCOc1cccc(c1)-c1cn([C@@H]2C[C@H](CN3CCC3)C2)c2ncnc(N)c12 |r,wD:16.16,18.19,(-.01,14.24,;.75,13.27,;.17,11.84,;1.12,10.63,;.54,9.2,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.55,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,)| Show InChI InChI=1S/C25H33N5O2/c1-2-9-31-10-11-32-21-6-3-5-19(14-21)22-16-30(25-23(22)24(26)27-17-28-25)20-12-18(13-20)15-29-7-4-8-29/h3,5-6,14,16-18,20H,2,4,7-13,15H2,1H3,(H2,26,27,28)/t18-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157883
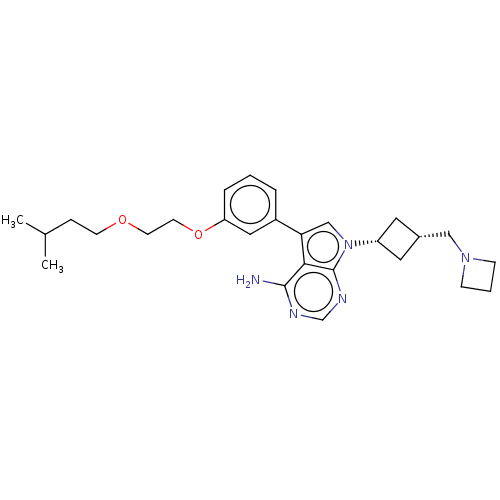
(CHEMBL3786115)Show SMILES CC(C)CCOCCOc1cccc(c1)-c1cn([C@@H]2C[C@H](CN3CCC3)C2)c2ncnc(N)c12 |r,wD:18.18,20.21,(.26,15.63,;-.2,14.48,;-1.42,14.31,;.75,13.27,;.17,11.84,;1.12,10.63,;.54,9.2,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.55,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,)| Show InChI InChI=1S/C27H37N5O2/c1-19(2)7-10-33-11-12-34-23-6-3-5-21(15-23)24-17-32(27-25(24)26(28)29-18-30-27)22-13-20(14-22)16-31-8-4-9-31/h3,5-6,15,17-20,22H,4,7-14,16H2,1-2H3,(H2,28,29,30)/t20-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157882
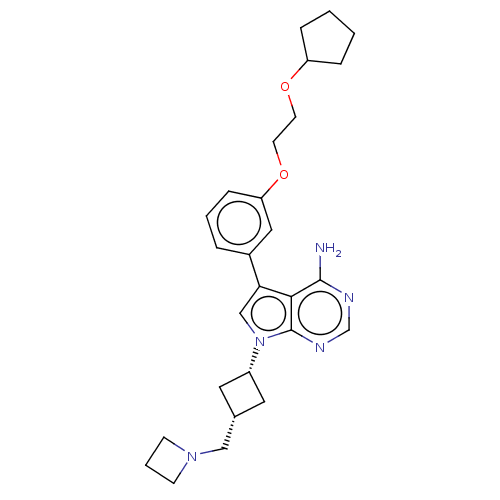
(CHEMBL3785169)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCCOC4CCCC4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:25.28,27.31,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;1.12,10.63,;.17,11.84,;.61,13.3,;-.67,14.16,;-1.88,13.21,;-1.36,11.77,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C27H35N5O2/c28-26-25-24(20-5-3-8-23(15-20)34-12-11-33-22-6-1-2-7-22)17-32(27(25)30-18-29-26)21-13-19(14-21)16-31-9-4-10-31/h3,5,8,15,17-19,21-22H,1-2,4,6-7,9-14,16H2,(H2,28,29,30)/t19-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157892
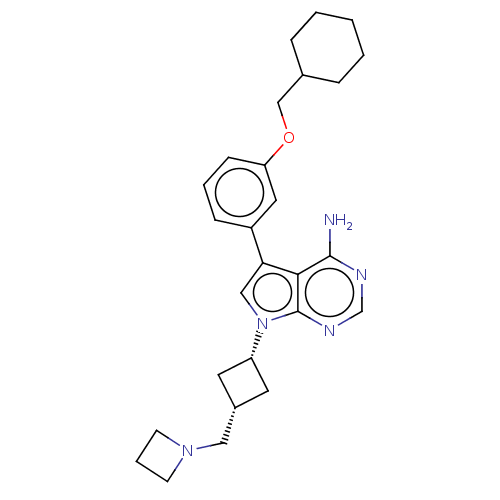
(CHEMBL3787399)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCC4CCCCC4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:24.27,26.30,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;1.12,10.63,;.17,11.84,;-1.36,11.62,;-1.93,10.19,;-.98,8.98,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C27H35N5O/c28-26-25-24(21-8-4-9-23(14-21)33-17-19-6-2-1-3-7-19)16-32(27(25)30-18-29-26)22-12-20(13-22)15-31-10-5-11-31/h4,8-9,14,16,18-20,22H,1-3,5-7,10-13,15,17H2,(H2,28,29,30)/t20-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157897
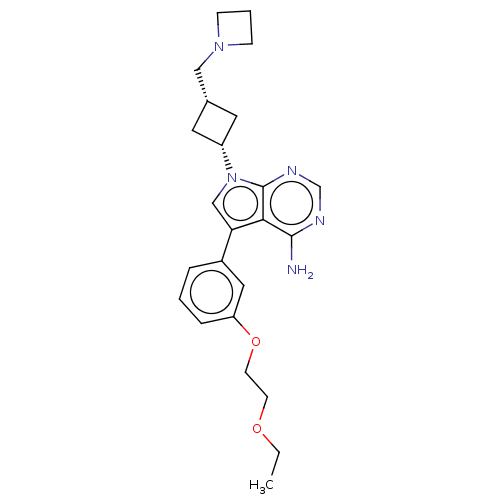
(CHEMBL3786668)Show SMILES CCOCCOc1cccc(c1)-c1cn([C@@H]2C[C@H](CN3CCC3)C2)c2ncnc(N)c12 |r,wD:15.15,17.18,(.63,12.98,;.17,11.84,;1.12,10.63,;.54,9.2,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.56,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,)| Show InChI InChI=1S/C24H31N5O2/c1-2-30-9-10-31-20-6-3-5-18(13-20)21-15-29(24-22(21)23(25)26-16-27-24)19-11-17(12-19)14-28-7-4-8-28/h3,5-6,13,15-17,19H,2,4,7-12,14H2,1H3,(H2,25,26,27)/t17-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50157878

(CHEMBL3786567)Show SMILES CC1(C)CC[C@@H](COc2cccc(c2)-c2cn([C@@H]3C[C@H](CN4CCC4)C3)c3ncnc(N)c23)O1 |r,wU:5.5,wD:17.17,19.20,(-1.8,11.77,;-1.51,10.57,;-2.39,9.7,;-.3,11.52,;.98,10.66,;.54,9.2,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.56,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,;-.98,9.12,)| Show InChI InChI=1S/C27H35N5O2/c1-27(2)8-7-22(34-27)16-33-21-6-3-5-19(13-21)23-15-32(26-24(23)25(28)29-17-30-26)20-11-18(12-20)14-31-9-4-10-31/h3,5-6,13,15,17-18,20,22H,4,7-12,14,16H2,1-2H3,(H2,28,29,30)/t18-,20+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157886
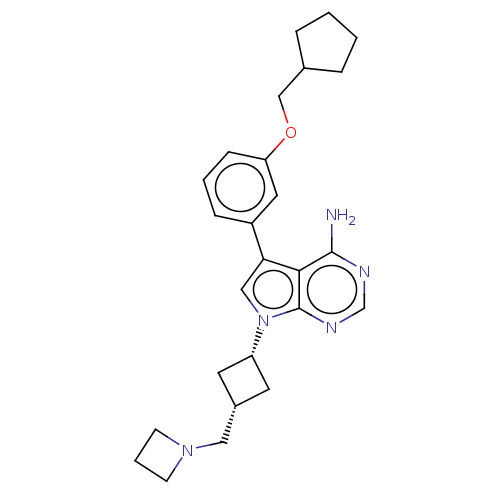
(CHEMBL3786564)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCC4CCCC4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:23.26,25.29,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;.98,10.66,;-.3,11.52,;-1.51,10.57,;-.98,9.12,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C26H33N5O/c27-25-24-23(20-7-3-8-22(13-20)32-16-18-5-1-2-6-18)15-31(26(24)29-17-28-25)21-11-19(12-21)14-30-9-4-10-30/h3,7-8,13,15,17-19,21H,1-2,4-6,9-12,14,16H2,(H2,27,28,29)/t19-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50157881

(CHEMBL3787614)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@@H]4CCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:23.26,16.15,25.29,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C25H31N5O2/c26-24-23-22(18-4-1-5-20(12-18)32-15-21-6-2-9-31-21)14-30(25(23)28-16-27-24)19-10-17(11-19)13-29-7-3-8-29/h1,4-5,12,14,16-17,19,21H,2-3,6-11,13,15H2,(H2,26,27,28)/t17-,19+,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Ins receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50157878

(CHEMBL3786567)Show SMILES CC1(C)CC[C@@H](COc2cccc(c2)-c2cn([C@@H]3C[C@H](CN4CCC4)C3)c3ncnc(N)c23)O1 |r,wU:5.5,wD:17.17,19.20,(-1.8,11.77,;-1.51,10.57,;-2.39,9.7,;-.3,11.52,;.98,10.66,;.54,9.2,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.56,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,;-.98,9.12,)| Show InChI InChI=1S/C27H35N5O2/c1-27(2)8-7-22(34-27)16-33-21-6-3-5-19(13-21)23-15-32(26-24(23)25(28)29-17-30-26)20-11-18(12-20)14-31-9-4-10-31/h3,5-6,13,15,17-18,20,22H,4,7-12,14,16H2,1-2H3,(H2,28,29,30)/t18-,20+,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Ret (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50157879

(CHEMBL1614712)Show SMILES [H][C@]1(CN2CCC2)C[C@]([H])(C1)n1cc(-c2cccc(OCc3ccccc3)c2)c2c(N)ncnc12 |wD:8.9,1.0,(6.47,-15.11,;5.71,-13.78,;4.93,-15.11,;5.69,-16.44,;5.68,-17.98,;7.22,-17.99,;7.23,-16.45,;5.31,-12.3,;6.81,-11.9,;6.02,-10.55,;7.2,-13.39,;7.59,-10.56,;6.61,-9.37,;7.45,-8.07,;7.04,-6.58,;5.56,-6.19,;5.15,-4.71,;6.24,-3.6,;7.73,-3.99,;8.81,-2.89,;10.15,-3.66,;11.48,-2.89,;12.81,-3.66,;14.12,-2.9,;14.13,-1.36,;12.8,-.59,;11.48,-1.36,;8.13,-5.47,;8.94,-8.47,;10.35,-7.7,;10.34,-6.15,;11.69,-8.46,;11.69,-10.01,;10.35,-10.79,;9.02,-10.01,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(21-8-4-9-23(14-21)33-17-19-6-2-1-3-7-19)16-32(27(25)30-18-29-26)22-12-20(13-22)15-31-10-5-11-31/h1-4,6-9,14,16,18,20,22H,5,10-13,15,17H2,(H2,28,29,30)/t20-,22+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Ins receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-alpha (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157885
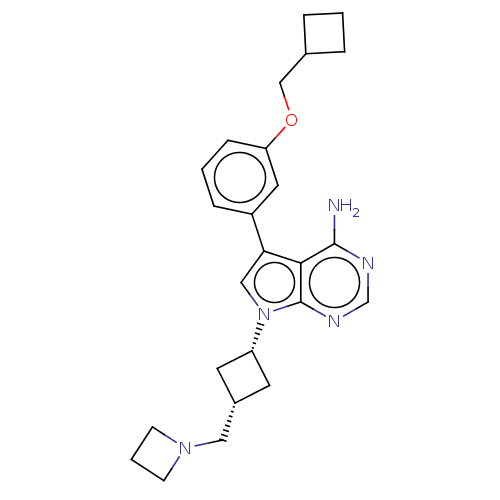
(CHEMBL3787253)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCC4CCC4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:22.25,24.28,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;.76,10.68,;-.77,10.87,;-.96,9.34,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C25H31N5O/c26-24-23-22(19-6-2-7-21(12-19)31-15-17-4-1-5-17)14-30(25(23)28-16-27-24)20-10-18(11-20)13-29-8-3-9-29/h2,6-7,12,14,16-18,20H,1,3-5,8-11,13,15H2,(H2,26,27,28)/t18-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157893
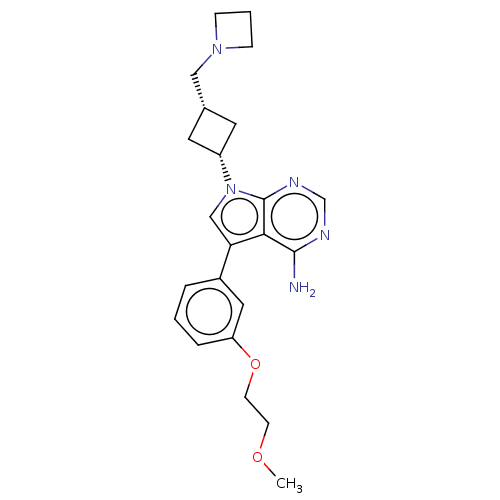
(CHEMBL3787579)Show SMILES COCCOc1cccc(c1)-c1cn([C@@H]2C[C@H](CN3CCC3)C2)c2ncnc(N)c12 |r,wD:14.14,16.17,(.36,11.6,;1.12,10.63,;.54,9.2,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.56,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,)| Show InChI InChI=1S/C23H29N5O2/c1-29-8-9-30-19-5-2-4-17(12-19)20-14-28(23-21(20)22(24)25-15-26-23)18-10-16(11-18)13-27-6-3-7-27/h2,4-5,12,14-16,18H,3,6-11,13H2,1H3,(H2,24,25,26)/t16-,18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Tested for neuronal nicotinic acetylcholine receptor (nAChR) binding in a whole rat brain preparation using [3H]-cystine as the radioligand. |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50157881

(CHEMBL3787614)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@@H]4CCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:23.26,16.15,25.29,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C25H31N5O2/c26-24-23-22(18-4-1-5-20(12-18)32-15-21-6-2-9-31-21)14-30(25(23)28-16-27-24)19-10-17(11-19)13-29-7-3-8-29/h1,4-5,12,14,16-17,19,21H,2-3,6-11,13,15H2,(H2,26,27,28)/t17-,19+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50157879

(CHEMBL1614712)Show SMILES [H][C@]1(CN2CCC2)C[C@]([H])(C1)n1cc(-c2cccc(OCc3ccccc3)c2)c2c(N)ncnc12 |wD:8.9,1.0,(6.47,-15.11,;5.71,-13.78,;4.93,-15.11,;5.69,-16.44,;5.68,-17.98,;7.22,-17.99,;7.23,-16.45,;5.31,-12.3,;6.81,-11.9,;6.02,-10.55,;7.2,-13.39,;7.59,-10.56,;6.61,-9.37,;7.45,-8.07,;7.04,-6.58,;5.56,-6.19,;5.15,-4.71,;6.24,-3.6,;7.73,-3.99,;8.81,-2.89,;10.15,-3.66,;11.48,-2.89,;12.81,-3.66,;14.12,-2.9,;14.13,-1.36,;12.8,-.59,;11.48,-1.36,;8.13,-5.47,;8.94,-8.47,;10.35,-7.7,;10.34,-6.15,;11.69,-8.46,;11.69,-10.01,;10.35,-10.79,;9.02,-10.01,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(21-8-4-9-23(14-21)33-17-19-6-2-1-3-7-19)16-32(27(25)30-18-29-26)22-12-20(13-22)15-31-10-5-11-31/h1-4,6-9,14,16,18,20,22H,5,10-13,15,17H2,(H2,28,29,30)/t20-,22+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Ret (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50157881

(CHEMBL3787614)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@@H]4CCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:23.26,16.15,25.29,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C25H31N5O2/c26-24-23-22(18-4-1-5-20(12-18)32-15-21-6-2-9-31-21)14-30(25(23)28-16-27-24)19-10-17(11-19)13-29-7-3-8-29/h1,4-5,12,14,16-17,19,21H,2-3,6-11,13,15H2,(H2,26,27,28)/t17-,19+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by dofetilide binding assay |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157891

(CHEMBL3785519)Show SMILES CC(Oc1cccc(c1)-c1cn([C@@H]2C[C@H](CN3CCC3)C2)c2ncnc(N)c12)c1ccccc1 |r,wD:12.12,14.15,(2.71,8.15,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.55,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,;.54,9.2,;1.12,10.63,;.17,11.84,;-1.36,11.62,;-1.93,10.19,;-.98,8.98,)| Show InChI InChI=1S/C28H31N5O/c1-19(21-7-3-2-4-8-21)34-24-10-5-9-22(15-24)25-17-33(28-26(25)27(29)30-18-31-28)23-13-20(14-23)16-32-11-6-12-32/h2-5,7-10,15,17-20,23H,6,11-14,16H2,1H3,(H2,29,30,31)/t19?,20-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50157880

(CHEMBL3787112)Show SMILES Nc1ncnc2n(cc(-c3cccc(O)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:17.19,19.22,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;1.1,6.31,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C20H23N5O/c21-19-18-17(14-3-1-4-16(26)9-14)11-25(20(18)23-12-22-19)15-7-13(8-15)10-24-5-2-6-24/h1,3-4,9,11-13,15,26H,2,5-8,10H2,(H2,21,22,23)/t13-,15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Ins receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50157881

(CHEMBL3787614)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@@H]4CCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:23.26,16.15,25.29,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C25H31N5O2/c26-24-23-22(18-4-1-5-20(12-18)32-15-21-6-2-9-31-21)14-30(25(23)28-16-27-24)19-10-17(11-19)13-29-7-3-8-29/h1,4-5,12,14,16-17,19,21H,2-3,6-11,13,15H2,(H2,26,27,28)/t17-,19+,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Ret (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50157879

(CHEMBL1614712)Show SMILES [H][C@]1(CN2CCC2)C[C@]([H])(C1)n1cc(-c2cccc(OCc3ccccc3)c2)c2c(N)ncnc12 |wD:8.9,1.0,(6.47,-15.11,;5.71,-13.78,;4.93,-15.11,;5.69,-16.44,;5.68,-17.98,;7.22,-17.99,;7.23,-16.45,;5.31,-12.3,;6.81,-11.9,;6.02,-10.55,;7.2,-13.39,;7.59,-10.56,;6.61,-9.37,;7.45,-8.07,;7.04,-6.58,;5.56,-6.19,;5.15,-4.71,;6.24,-3.6,;7.73,-3.99,;8.81,-2.89,;10.15,-3.66,;11.48,-2.89,;12.81,-3.66,;14.12,-2.9,;14.13,-1.36,;12.8,-.59,;11.48,-1.36,;8.13,-5.47,;8.94,-8.47,;10.35,-7.7,;10.34,-6.15,;11.69,-8.46,;11.69,-10.01,;10.35,-10.79,;9.02,-10.01,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(21-8-4-9-23(14-21)33-17-19-6-2-1-3-7-19)16-32(27(25)30-18-29-26)22-12-20(13-22)15-31-10-5-11-31/h1-4,6-9,14,16,18,20,22H,5,10-13,15,17H2,(H2,28,29,30)/t20-,22+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50157881

(CHEMBL3787614)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@@H]4CCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:23.26,16.15,25.29,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C25H31N5O2/c26-24-23-22(18-4-1-5-20(12-18)32-15-21-6-2-9-31-21)14-30(25(23)28-16-27-24)19-10-17(11-19)13-29-7-3-8-29/h1,4-5,12,14,16-17,19,21H,2-3,6-11,13,15H2,(H2,26,27,28)/t17-,19+,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50157879

(CHEMBL1614712)Show SMILES [H][C@]1(CN2CCC2)C[C@]([H])(C1)n1cc(-c2cccc(OCc3ccccc3)c2)c2c(N)ncnc12 |wD:8.9,1.0,(6.47,-15.11,;5.71,-13.78,;4.93,-15.11,;5.69,-16.44,;5.68,-17.98,;7.22,-17.99,;7.23,-16.45,;5.31,-12.3,;6.81,-11.9,;6.02,-10.55,;7.2,-13.39,;7.59,-10.56,;6.61,-9.37,;7.45,-8.07,;7.04,-6.58,;5.56,-6.19,;5.15,-4.71,;6.24,-3.6,;7.73,-3.99,;8.81,-2.89,;10.15,-3.66,;11.48,-2.89,;12.81,-3.66,;14.12,-2.9,;14.13,-1.36,;12.8,-.59,;11.48,-1.36,;8.13,-5.47,;8.94,-8.47,;10.35,-7.7,;10.34,-6.15,;11.69,-8.46,;11.69,-10.01,;10.35,-10.79,;9.02,-10.01,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(21-8-4-9-23(14-21)33-17-19-6-2-1-3-7-19)16-32(27(25)30-18-29-26)22-12-20(13-22)15-31-10-5-11-31/h1-4,6-9,14,16,18,20,22H,5,10-13,15,17H2,(H2,28,29,30)/t20-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50157878

(CHEMBL3786567)Show SMILES CC1(C)CC[C@@H](COc2cccc(c2)-c2cn([C@@H]3C[C@H](CN4CCC4)C3)c3ncnc(N)c23)O1 |r,wU:5.5,wD:17.17,19.20,(-1.8,11.77,;-1.51,10.57,;-2.39,9.7,;-.3,11.52,;.98,10.66,;.54,9.2,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.56,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,;-.98,9.12,)| Show InChI InChI=1S/C27H35N5O2/c1-27(2)8-7-22(34-27)16-33-21-6-3-5-19(13-21)23-15-32(26-24(23)25(28)29-17-30-26)20-11-18(12-20)14-31-9-4-10-31/h3,5-6,13,15,17-18,20,22H,4,7-12,14,16H2,1-2H3,(H2,28,29,30)/t18-,20+,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Tested for neuronal nicotinic acetylcholine receptor (nAChR) binding in a whole rat brain preparation using [3H]-cystine as the radioligand. |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157896
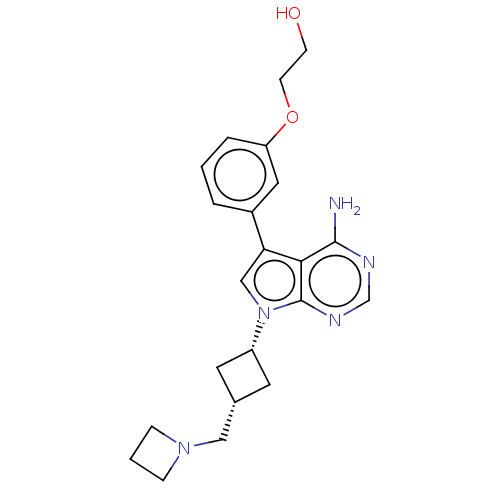
(CHEMBL3787474)Show SMILES Nc1ncnc2n(cc(-c3cccc(OCCO)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:20.22,22.25,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.56,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;1,10.34,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C22H27N5O2/c23-21-20-19(16-3-1-4-18(11-16)29-8-7-28)13-27(22(20)25-14-24-21)17-9-15(10-17)12-26-5-2-6-26/h1,3-4,11,13-15,17,28H,2,5-10,12H2,(H2,23,24,25)/t15-,17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50157881

(CHEMBL3787614)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@@H]4CCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:23.26,16.15,25.29,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C25H31N5O2/c26-24-23-22(18-4-1-5-20(12-18)32-15-21-6-2-9-31-21)14-30(25(23)28-16-27-24)19-10-17(11-19)13-29-7-3-8-29/h1,4-5,12,14,16-17,19,21H,2-3,6-11,13,15H2,(H2,26,27,28)/t17-,19+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Lck (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50157879

(CHEMBL1614712)Show SMILES [H][C@]1(CN2CCC2)C[C@]([H])(C1)n1cc(-c2cccc(OCc3ccccc3)c2)c2c(N)ncnc12 |wD:8.9,1.0,(6.47,-15.11,;5.71,-13.78,;4.93,-15.11,;5.69,-16.44,;5.68,-17.98,;7.22,-17.99,;7.23,-16.45,;5.31,-12.3,;6.81,-11.9,;6.02,-10.55,;7.2,-13.39,;7.59,-10.56,;6.61,-9.37,;7.45,-8.07,;7.04,-6.58,;5.56,-6.19,;5.15,-4.71,;6.24,-3.6,;7.73,-3.99,;8.81,-2.89,;10.15,-3.66,;11.48,-2.89,;12.81,-3.66,;14.12,-2.9,;14.13,-1.36,;12.8,-.59,;11.48,-1.36,;8.13,-5.47,;8.94,-8.47,;10.35,-7.7,;10.34,-6.15,;11.69,-8.46,;11.69,-10.01,;10.35,-10.79,;9.02,-10.01,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(21-8-4-9-23(14-21)33-17-19-6-2-1-3-7-19)16-32(27(25)30-18-29-26)22-12-20(13-22)15-31-10-5-11-31/h1-4,6-9,14,16,18,20,22H,5,10-13,15,17H2,(H2,28,29,30)/t20-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-beta (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50157881

(CHEMBL3787614)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@@H]4CCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:23.26,16.15,25.29,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C25H31N5O2/c26-24-23-22(18-4-1-5-20(12-18)32-15-21-6-2-9-31-21)14-30(25(23)28-16-27-24)19-10-17(11-19)13-29-7-3-8-29/h1,4-5,12,14,16-17,19,21H,2-3,6-11,13,15H2,(H2,26,27,28)/t17-,19+,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-beta (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50157878

(CHEMBL3786567)Show SMILES CC1(C)CC[C@@H](COc2cccc(c2)-c2cn([C@@H]3C[C@H](CN4CCC4)C3)c3ncnc(N)c23)O1 |r,wU:5.5,wD:17.17,19.20,(-1.8,11.77,;-1.51,10.57,;-2.39,9.7,;-.3,11.52,;.98,10.66,;.54,9.2,;1.49,7.98,;.91,6.55,;1.86,5.34,;3.38,5.56,;4.33,4.34,;3.76,2.91,;2.23,2.7,;1.29,3.91,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,;.3,-.77,;-1.03,-1.55,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;-1.03,2.79,;.3,.77,;-.98,9.12,)| Show InChI InChI=1S/C27H35N5O2/c1-27(2)8-7-22(34-27)16-33-21-6-3-5-19(13-21)23-15-32(26-24(23)25(28)29-17-30-26)20-11-18(12-20)14-31-9-4-10-31/h3,5-6,13,15,17-18,20,22H,4,7-12,14,16H2,1-2H3,(H2,28,29,30)/t18-,20+,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-beta (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50157881

(CHEMBL3787614)Show SMILES Nc1ncnc2n(cc(-c3cccc(OC[C@@H]4CCCO4)c3)c12)[C@@H]1C[C@H](CN2CCC2)C1 |r,wD:23.26,16.15,25.29,(-1.03,2.79,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.66,.02,;1.76,1.24,;2.23,2.7,;3.76,2.91,;4.33,4.34,;3.38,5.55,;1.86,5.34,;.91,6.55,;1.49,7.98,;.54,9.2,;-.98,9.12,;-1.51,10.57,;-.3,11.52,;.98,10.66,;1.29,3.91,;.3,.77,;2.24,-2.7,;3.54,-3.45,;2.74,-4.76,;3.1,-6.25,;4.58,-6.69,;5.27,-8.02,;6.62,-7.28,;5.88,-5.93,;1.42,-3.96,)| Show InChI InChI=1S/C25H31N5O2/c26-24-23-22(18-4-1-5-20(12-18)32-15-21-6-2-9-31-21)14-30(25(23)28-16-27-24)19-10-17(11-19)13-29-7-3-8-29/h1,4-5,12,14,16-17,19,21H,2-3,6-11,13,15H2,(H2,26,27,28)/t17-,19+,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-alpha (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50157879

(CHEMBL1614712)Show SMILES [H][C@]1(CN2CCC2)C[C@]([H])(C1)n1cc(-c2cccc(OCc3ccccc3)c2)c2c(N)ncnc12 |wD:8.9,1.0,(6.47,-15.11,;5.71,-13.78,;4.93,-15.11,;5.69,-16.44,;5.68,-17.98,;7.22,-17.99,;7.23,-16.45,;5.31,-12.3,;6.81,-11.9,;6.02,-10.55,;7.2,-13.39,;7.59,-10.56,;6.61,-9.37,;7.45,-8.07,;7.04,-6.58,;5.56,-6.19,;5.15,-4.71,;6.24,-3.6,;7.73,-3.99,;8.81,-2.89,;10.15,-3.66,;11.48,-2.89,;12.81,-3.66,;14.12,-2.9,;14.13,-1.36,;12.8,-.59,;11.48,-1.36,;8.13,-5.47,;8.94,-8.47,;10.35,-7.7,;10.34,-6.15,;11.69,-8.46,;11.69,-10.01,;10.35,-10.79,;9.02,-10.01,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(21-8-4-9-23(14-21)33-17-19-6-2-1-3-7-19)16-32(27(25)30-18-29-26)22-12-20(13-22)15-31-10-5-11-31/h1-4,6-9,14,16,18,20,22H,5,10-13,15,17H2,(H2,28,29,30)/t20-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-alpha (unknown origin) |
Bioorg Med Chem Lett 26: 2065-7 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.074
BindingDB Entry DOI: 10.7270/Q21Z469M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data