

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50180683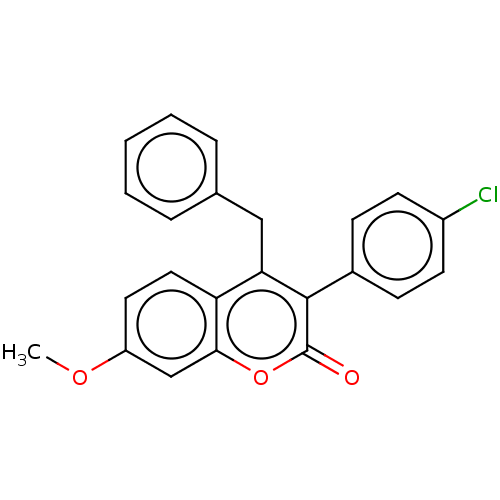 (CHEMBL3818720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Competitive inhibition of human placental microsomal aromatase using androgen as substrate | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) transfected in human MCF7 cells | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50123026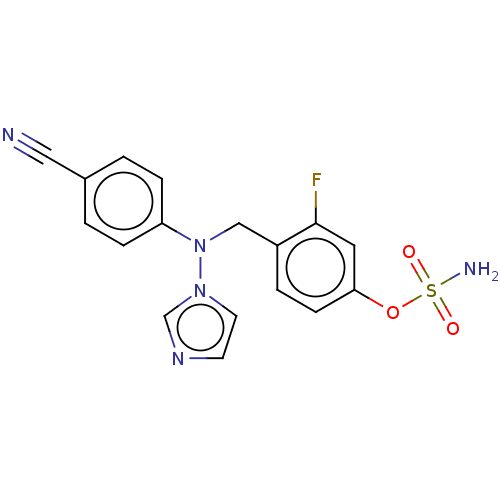 (CHEMBL3623232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of aromatase in human JEG3 cells using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 1 hr by scintillati... | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50180685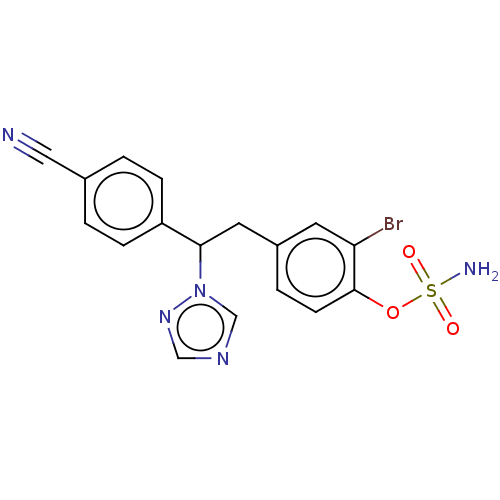 (CHEMBL3818343) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of aromatase in human JEG3 cells using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 1 hr by scintillati... | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50014315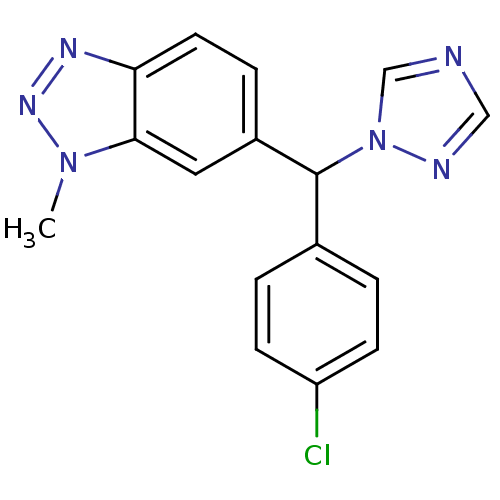 ((rac)-6-((4-chlorophenyl)(1H-1,2,4-triazol-1-yl)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Reversible inhibition of aromatase (unknown origin) | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10015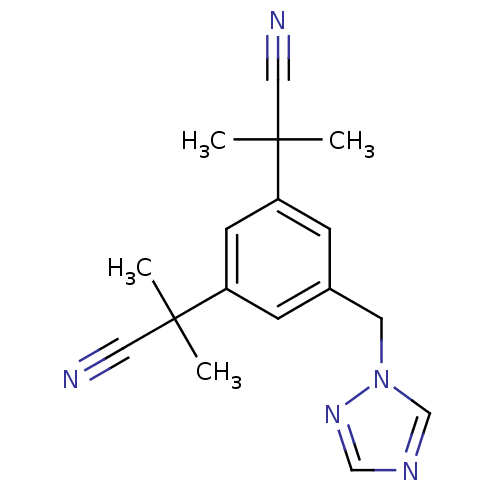 (2-[3-(1-cyano-1-methylethyl)-5-(1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) transfected in human MCF7 cells | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50435004 (CHEMBL2386284) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human placental microsomal aromatase using testosterone as substrate assessed as formation of estradiol after 10 mins | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50240798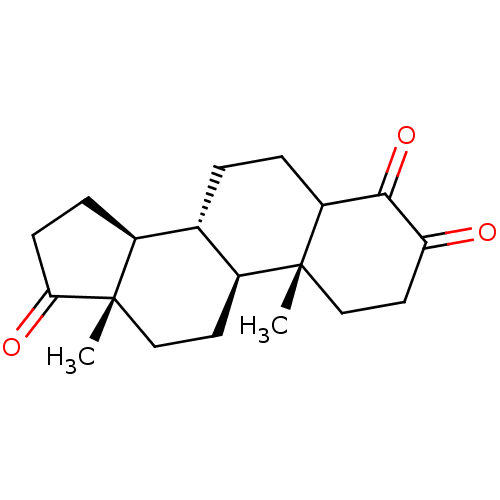 ((8R,9S,10R,13S,14S)-4-Hydroxy-10,13-dimethyl-1,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of particulate fractions of human breast cancer derived aromatase | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50180688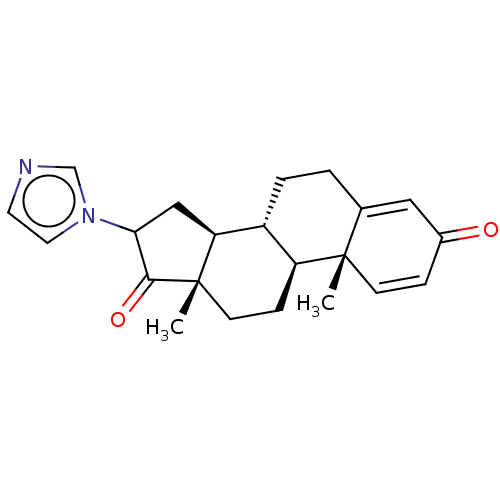 (CHEMBL3818725) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release preincubated for 5... | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50123012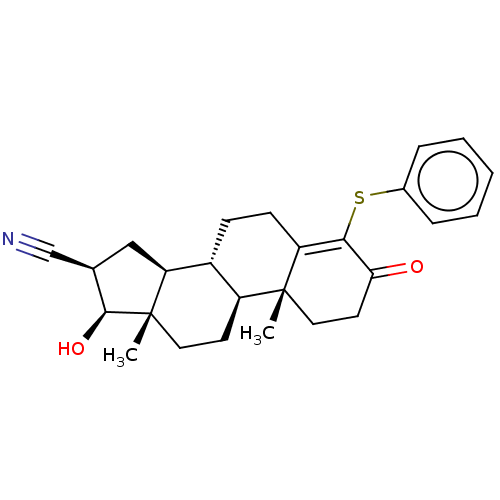 (CHEMBL3623217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 5 mins by be... | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50180689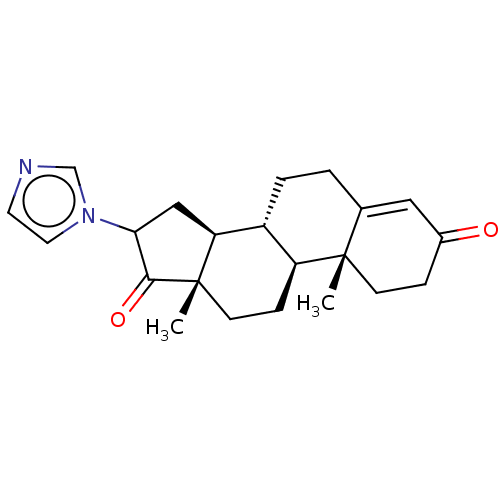 (CHEMBL3819184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release preincubated for 5... | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50180690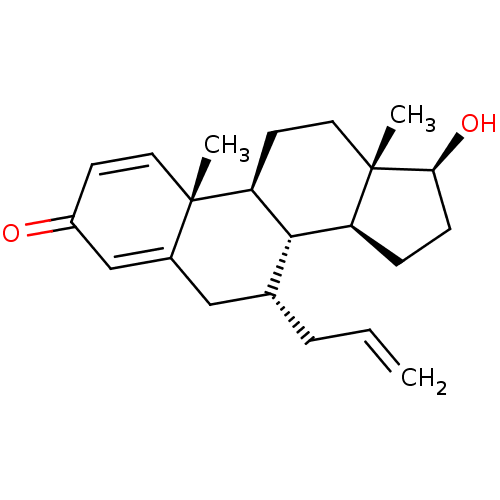 (CHEMBL3819549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 15 mins by l... | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50123151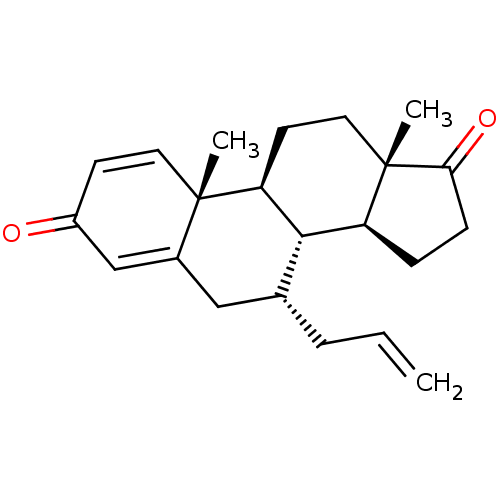 (CHEMBL3623223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 15 mins by l... | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50180687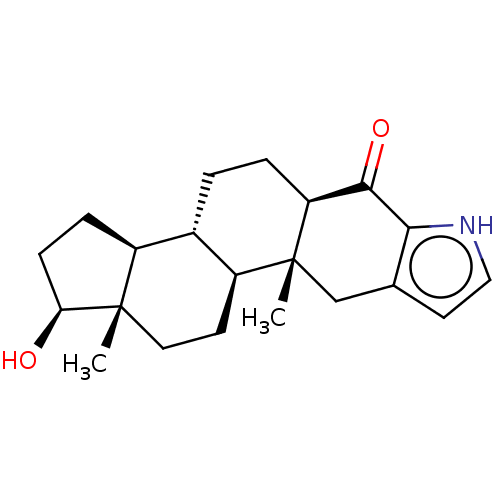 (CHEMBL3818442) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 512 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release preincubated for 5... | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50180692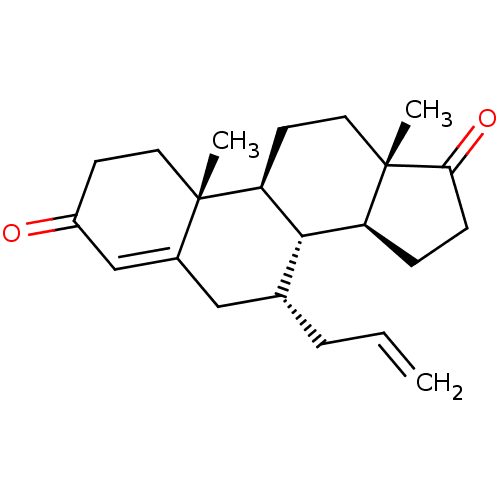 (CHEMBL3818728) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 15 mins by l... | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50180691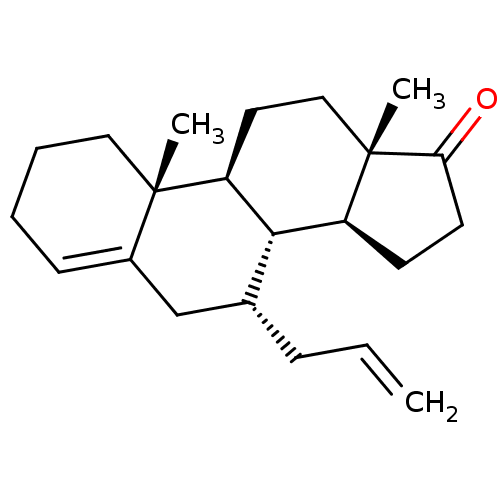 (CHEMBL3818338) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release after 15 mins by l... | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50180686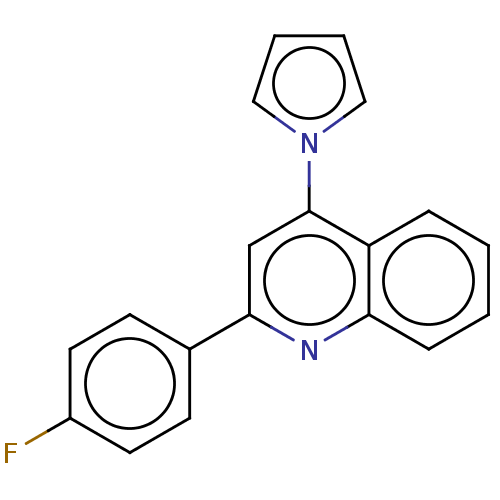 (CHEMBL3818279) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human aromatase using testosterone as substrate incubated for 1 hr by HTRF assay | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50123013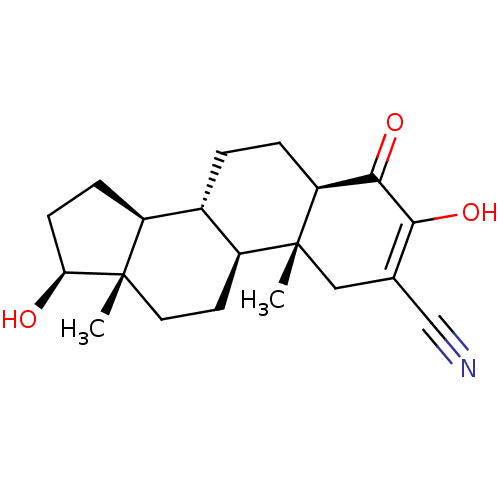 (CHEMBL3623216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human placental microsomal aromatase using [1beta-3H] androstenedione as substrate assessed as tritiated H2O release preincubated for 5... | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50435003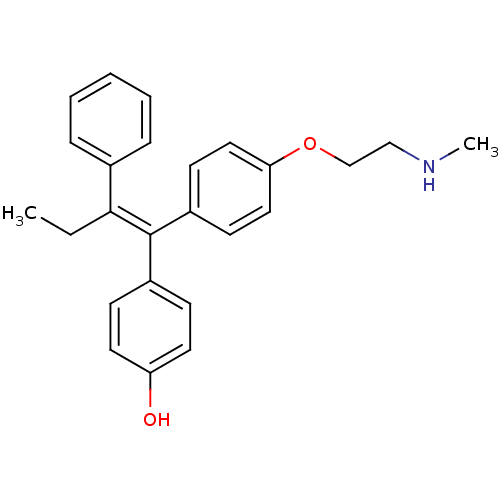 (ENDOXIFEN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human placental microsomal aromatase using testosterone as substrate assessed as formation of estradiol after 10 mins | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human aromatase transfected in human MCF7 cells | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50180684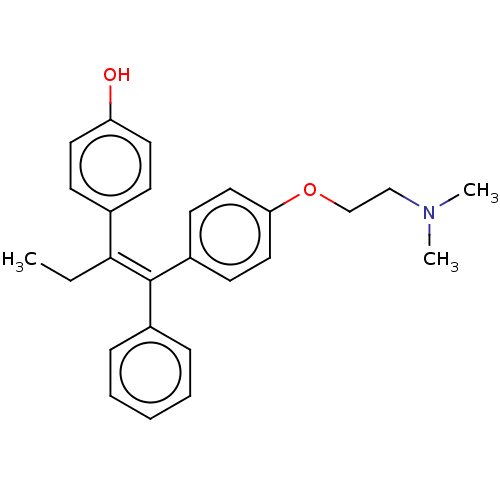 (4-OHT | Afimoxifene | TamoGel) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Upstate Medical University Curated by ChEMBL | Assay Description Inhibition of human placental microsomal aromatase using testosterone as substrate assessed as formation of estradiol after 10 mins | J Med Chem 59: 5131-48 (2016) Article DOI: 10.1021/acs.jmedchem.5b01281 BindingDB Entry DOI: 10.7270/Q2TM7D2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||