

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318887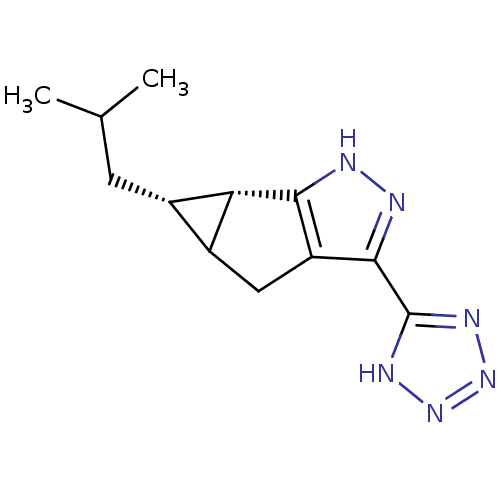 ((5S,5aS)-5-isobutyl-3-(1H-tetrazol-5-yl)-4,4a,5,5a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318904 ((4aR,5R,5aS)-5-allyl-3-(1H-tetrazol-5-yl)-4,4a,5,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318889 ((4aR,5R,5aS)-5-butyl-3-(1H-tetrazol-5-yl)-4,4a,5,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.75E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318890 ((4aS,5R,5aR)-5-(methoxymethyl)-3-(1H-tetrazol-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318891 ((4aS,5aR)-5,5-dimethyl-3-(1H-tetrazol-5-yl)-4,4a,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.86E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318892 ((2'R,4'S)-7'-(1H-1,2,3,4-tetrazol-5-yl)-8',9'-diaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50273099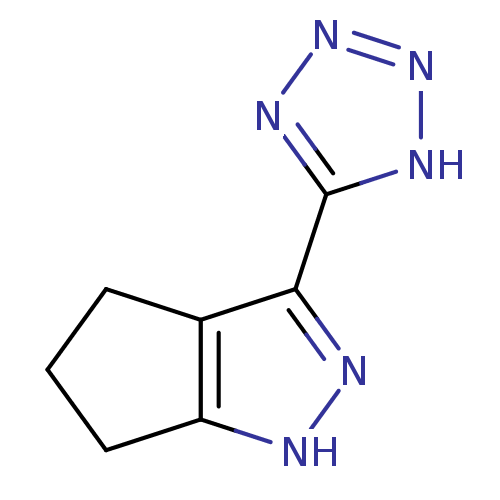 (3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydrocyclopenta[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 286 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318893 ((+/-)-3-(1H-tetrazol-5-yl)-4,4a,5,5a-tetrahydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318894 ((+)-3-(1H-tetrazol-5-yl)-4,4a,5,5a-tetrahydro-1H-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318895 ((1S,1aS,5aR)-1-Methyl-4-(1H-tetrazol-5-yl)-1a,2,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318896 ((1R,1aS,5aR)-1-Methyl-4-(1H-tetrazol-5-yl)-1a,2,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 244 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318897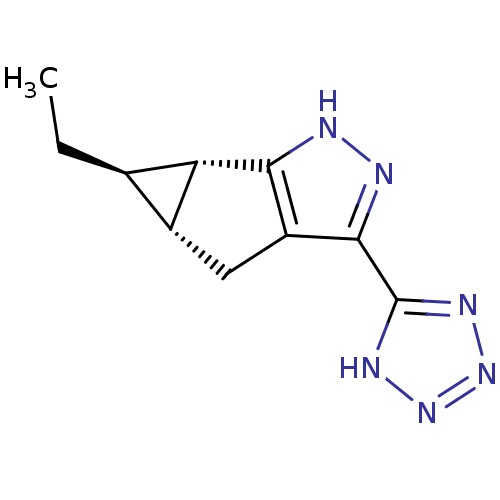 ((4aR,5R,5aS)-5-ethyl-3-(1H-tetrazol-5-yl)-4,4a,5,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 504 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318898 ((4aR,5S,5aR)-3-(1H-tetrazol-5-yl)-5-vinyl-4,4a,5,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318899 ((4aR,5R,5aR)-3-(1H-tetrazol-5-yl)-5-vinyl-4,4a,5,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 156 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318900 ((4aR,5R,5aR)-5-cyclopropyl-3-(1H-tetrazol-5-yl)-4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 507 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318901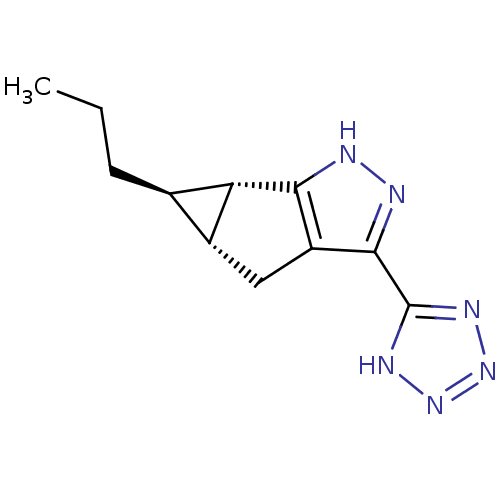 ((1R,1aS,5aR)-1-Propyl-4-(1H-tetrazol-5-yl)-1a,2,5,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 541 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318902 ((4aR,5R,5aR)-5-[(1E)-prop-1-enyl]-3-(1H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 276 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318903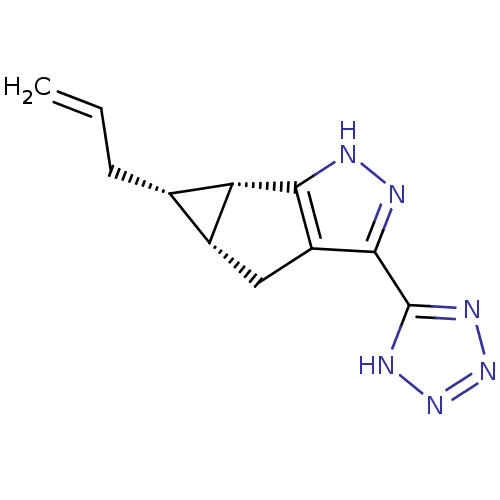 ((4aR,5S,5aS)-5-allyl-3-(1H-tetrazol-5-yl)-4,4a,5,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50318888 ((5R,5aS)-5-isobutyl-3-(1H-tetrazol-5-yl)-4,4a,5,5a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GPR109a expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production by HTRF assay | Bioorg Med Chem Lett 20: 2797-800 (2010) Article DOI: 10.1016/j.bmcl.2010.03.062 BindingDB Entry DOI: 10.7270/Q2X0676V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||