 Found 155 hits Enz. Inhib. hit(s) with all data for entry = 50033632
Found 155 hits Enz. Inhib. hit(s) with all data for entry = 50033632 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM29241
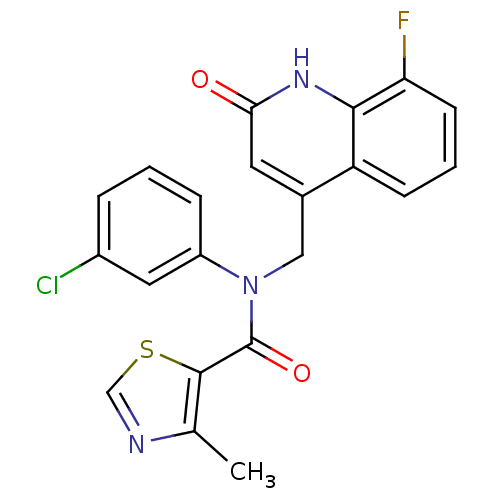
(quinolinone, 12)Show SMILES Cc1ncsc1C(=O)N(Cc1cc(=O)[nH]c2c(F)cccc12)c1cccc(Cl)c1 Show InChI InChI=1S/C21H15ClFN3O2S/c1-12-20(29-11-24-12)21(28)26(15-5-2-4-14(22)9-15)10-13-8-18(27)25-19-16(13)6-3-7-17(19)23/h2-9,11H,10H2,1H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50348709
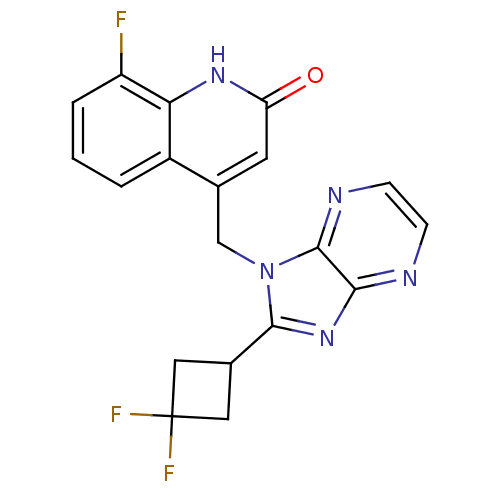
(CHEMBL1801062)Show SMILES Fc1cccc2c(Cn3c(nc4nccnc34)C3CC(F)(F)C3)cc(=O)[nH]c12 Show InChI InChI=1S/C19H14F3N5O/c20-13-3-1-2-12-10(6-14(28)25-15(12)13)9-27-17(11-7-19(21,22)8-11)26-16-18(27)24-5-4-23-16/h1-6,11H,7-9H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50348709

(CHEMBL1801062)Show SMILES Fc1cccc2c(Cn3c(nc4nccnc34)C3CC(F)(F)C3)cc(=O)[nH]c12 Show InChI InChI=1S/C19H14F3N5O/c20-13-3-1-2-12-10(6-14(28)25-15(12)13)9-27-17(11-7-19(21,22)8-11)26-16-18(27)24-5-4-23-16/h1-6,11H,7-9H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50348709

(CHEMBL1801062)Show SMILES Fc1cccc2c(Cn3c(nc4nccnc34)C3CC(F)(F)C3)cc(=O)[nH]c12 Show InChI InChI=1S/C19H14F3N5O/c20-13-3-1-2-12-10(6-14(28)25-15(12)13)9-27-17(11-7-19(21,22)8-11)26-16-18(27)24-5-4-23-16/h1-6,11H,7-9H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50348710
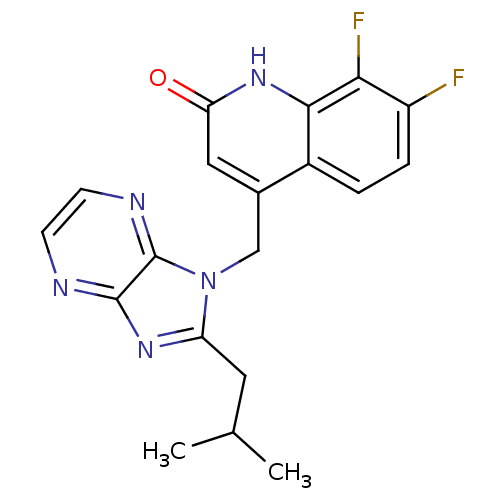
(CHEMBL1801506)Show SMILES CC(C)Cc1nc2nccnc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C19H17F2N5O/c1-10(2)7-14-24-18-19(23-6-5-22-18)26(14)9-11-8-15(27)25-17-12(11)3-4-13(20)16(17)21/h3-6,8,10H,7,9H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50348709

(CHEMBL1801062)Show SMILES Fc1cccc2c(Cn3c(nc4nccnc34)C3CC(F)(F)C3)cc(=O)[nH]c12 Show InChI InChI=1S/C19H14F3N5O/c20-13-3-1-2-12-10(6-14(28)25-15(12)13)9-27-17(11-7-19(21,22)8-11)26-16-18(27)24-5-4-23-16/h1-6,11H,7-9H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM29239

(quinolinone, 10)Show SMILES Cc1ncsc1C(=O)N(Cc1cc(=O)[nH]c2c(F)cccc12)c1ccccc1 Show InChI InChI=1S/C21H16FN3O2S/c1-13-20(28-12-23-13)21(27)25(15-6-3-2-4-7-15)11-14-10-18(26)24-19-16(14)8-5-9-17(19)22/h2-10,12H,11H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS expressed in HEK293 cells assessed as inhibition ionomycin induced nitric oxide production incubated for 24 hrs measured aft... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50348711
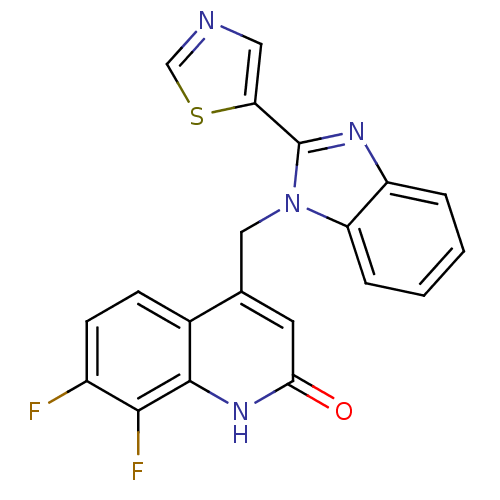
(CHEMBL1801475)Show SMILES Fc1ccc2c(Cn3c(nc4ccccc34)-c3cncs3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C20H12F2N4OS/c21-13-6-5-12-11(7-17(27)25-19(12)18(13)22)9-26-15-4-2-1-3-14(15)24-20(26)16-8-23-10-28-16/h1-8,10H,9H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS expressed in HEK293 cells assessed as inhibition ionomycin induced nitric oxide production incubated for 24 hrs measured aft... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50348712

(CHEMBL1801476)Show SMILES Cn1cncc1-c1nc2ccccc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C21H15F2N5O/c1-27-11-24-9-17(27)21-25-15-4-2-3-5-16(15)28(21)10-12-8-18(29)26-20-13(12)6-7-14(22)19(20)23/h2-9,11H,10H2,1H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS expressed in HEK293 cells assessed as inhibition ionomycin induced nitric oxide production incubated for 24 hrs measured aft... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50348713
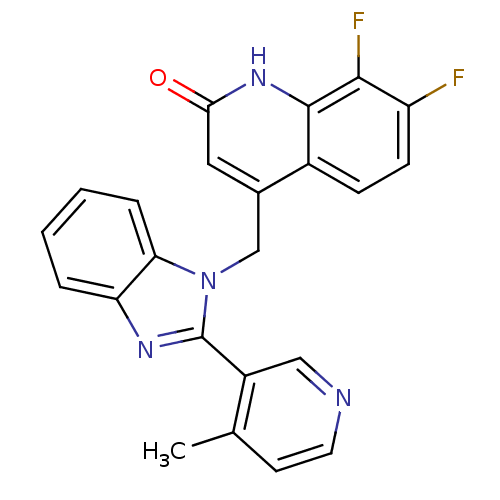
(CHEMBL1801477)Show SMILES Cc1ccncc1-c1nc2ccccc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C23H16F2N4O/c1-13-8-9-26-11-16(13)23-27-18-4-2-3-5-19(18)29(23)12-14-10-20(30)28-22-15(14)6-7-17(24)21(22)25/h2-11H,12H2,1H3,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS expressed in HEK293 cells assessed as inhibition ionomycin induced nitric oxide production incubated for 24 hrs measured aft... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50348714
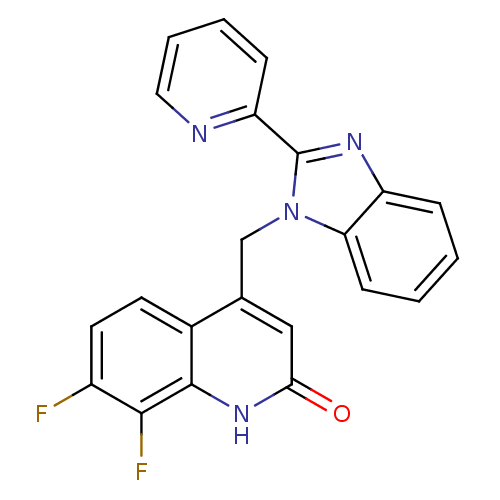
(CHEMBL1801478)Show SMILES Fc1ccc2c(Cn3c(nc4ccccc34)-c3ccccn3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C22H14F2N4O/c23-15-9-8-14-13(11-19(29)27-21(14)20(15)24)12-28-18-7-2-1-5-16(18)26-22(28)17-6-3-4-10-25-17/h1-11H,12H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS expressed in HEK293 cells assessed as inhibition ionomycin induced nitric oxide production incubated for 24 hrs measured aft... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50348715
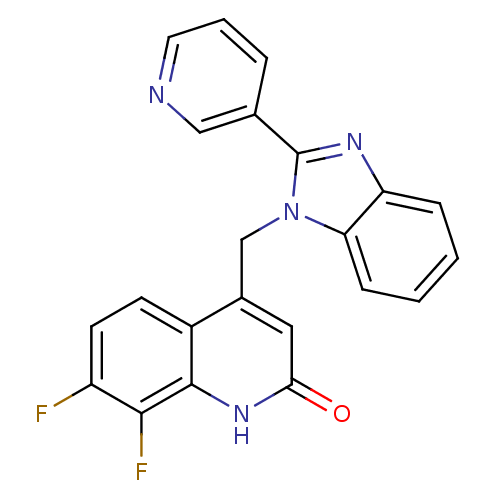
(CHEMBL1801479)Show SMILES Fc1ccc2c(Cn3c(nc4ccccc34)-c3cccnc3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C22H14F2N4O/c23-16-8-7-15-14(10-19(29)27-21(15)20(16)24)12-28-18-6-2-1-5-17(18)26-22(28)13-4-3-9-25-11-13/h1-11H,12H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS expressed in HEK293 cells assessed as inhibition ionomycin induced nitric oxide production incubated for 24 hrs measured aft... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50348716
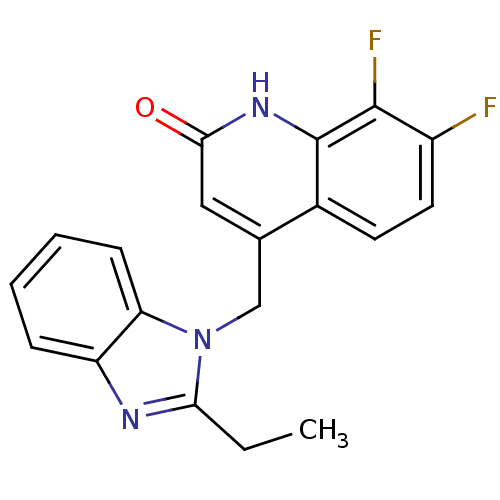
(CHEMBL1801483)Show SMILES CCc1nc2ccccc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C19H15F2N3O/c1-2-16-22-14-5-3-4-6-15(14)24(16)10-11-9-17(25)23-19-12(11)7-8-13(20)18(19)21/h3-9H,2,10H2,1H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS expressed in HEK293 cells assessed as inhibition ionomycin induced nitric oxide production incubated for 24 hrs measured aft... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50348717
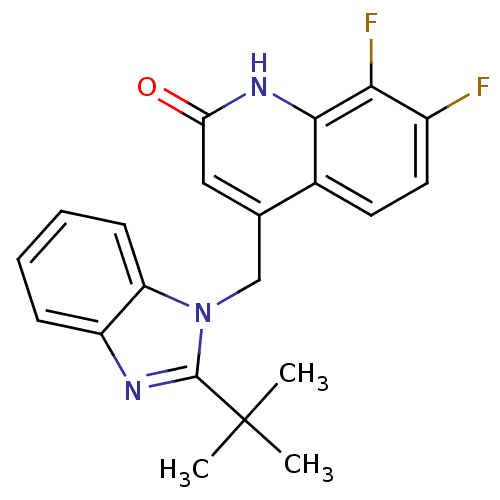
(CHEMBL1801484)Show SMILES CC(C)(C)c1nc2ccccc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C21H19F2N3O/c1-21(2,3)20-24-15-6-4-5-7-16(15)26(20)11-12-10-17(27)25-19-13(12)8-9-14(22)18(19)23/h4-10H,11H2,1-3H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS expressed in HEK293 cells assessed as inhibition ionomycin induced nitric oxide production incubated for 24 hrs measured aft... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50348718
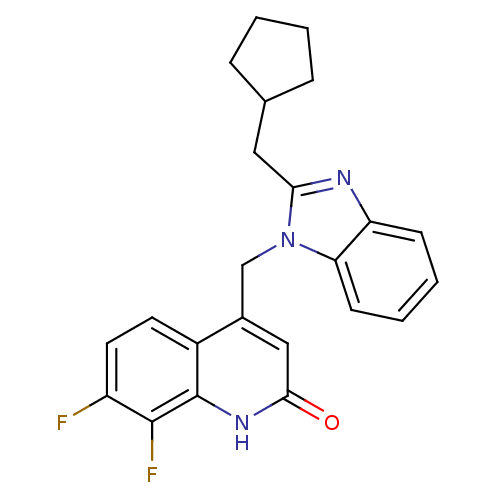
(CHEMBL1801494)Show SMILES Fc1ccc2c(Cn3c(CC4CCCC4)nc4ccccc34)cc(=O)[nH]c2c1F Show InChI InChI=1S/C23H21F2N3O/c24-17-10-9-16-15(12-21(29)27-23(16)22(17)25)13-28-19-8-4-3-7-18(19)26-20(28)11-14-5-1-2-6-14/h3-4,7-10,12,14H,1-2,5-6,11,13H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human nNOS expressed in HEK293 cells assessed as inhibition ionomycin induced nitric oxide production incubated for 24 hrs measured aft... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM29239

(quinolinone, 10)Show SMILES Cc1ncsc1C(=O)N(Cc1cc(=O)[nH]c2c(F)cccc12)c1ccccc1 Show InChI InChI=1S/C21H16FN3O2S/c1-13-20(28-12-23-13)21(27)25(15-6-3-2-4-7-15)11-14-10-18(26)24-19-16(14)8-5-9-17(19)22/h2-10,12H,11H2,1H3,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348711

(CHEMBL1801475)Show SMILES Fc1ccc2c(Cn3c(nc4ccccc34)-c3cncs3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C20H12F2N4OS/c21-13-6-5-12-11(7-17(27)25-19(12)18(13)22)9-26-15-4-2-1-3-14(15)24-20(26)16-8-23-10-28-16/h1-8,10H,9H2,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348713

(CHEMBL1801477)Show SMILES Cc1ccncc1-c1nc2ccccc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C23H16F2N4O/c1-13-8-9-26-11-16(13)23-27-18-4-2-3-5-19(18)29(23)12-14-10-20(30)28-22-15(14)6-7-17(24)21(22)25/h2-11H,12H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348714

(CHEMBL1801478)Show SMILES Fc1ccc2c(Cn3c(nc4ccccc34)-c3ccccn3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C22H14F2N4O/c23-15-9-8-14-13(11-19(29)27-21(14)20(15)24)12-28-18-7-2-1-5-16(18)26-22(28)17-6-3-4-10-25-17/h1-11H,12H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348715

(CHEMBL1801479)Show SMILES Fc1ccc2c(Cn3c(nc4ccccc34)-c3cccnc3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C22H14F2N4O/c23-16-8-7-15-14(10-19(29)27-21(15)20(16)24)12-28-18-6-2-1-5-17(18)26-22(28)13-4-3-9-25-11-13/h1-11H,12H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348717

(CHEMBL1801484)Show SMILES CC(C)(C)c1nc2ccccc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C21H19F2N3O/c1-21(2,3)20-24-15-6-4-5-7-16(15)26(20)11-12-10-17(27)25-19-13(12)8-9-14(22)18(19)23/h4-10H,11H2,1-3H3,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348719
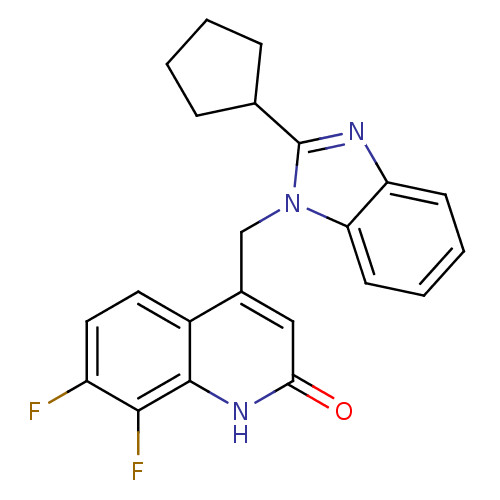
(CHEMBL1801488)Show SMILES Fc1ccc2c(Cn3c(nc4ccccc34)C3CCCC3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C22H19F2N3O/c23-16-10-9-15-14(11-19(28)26-21(15)20(16)24)12-27-18-8-4-3-7-17(18)25-22(27)13-5-1-2-6-13/h3-4,7-11,13H,1-2,5-6,12H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
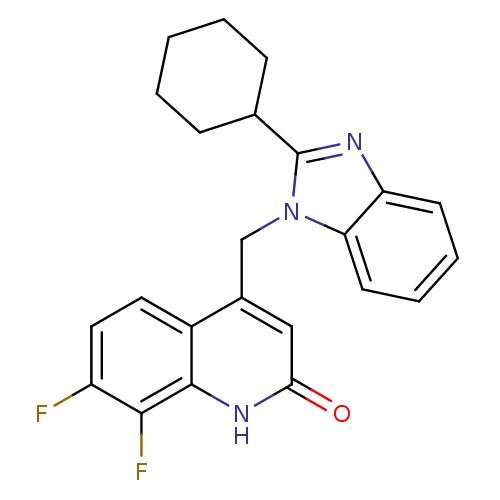
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348720
(CHEMBL1801489)Show SMILES Fc1ccc2c(Cn3c(nc4ccccc34)C3CCCCC3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C23H21F2N3O/c24-17-11-10-16-15(12-20(29)27-22(16)21(17)25)13-28-19-9-5-4-8-18(19)26-23(28)14-6-2-1-3-7-14/h4-5,8-12,14H,1-3,6-7,13H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348721
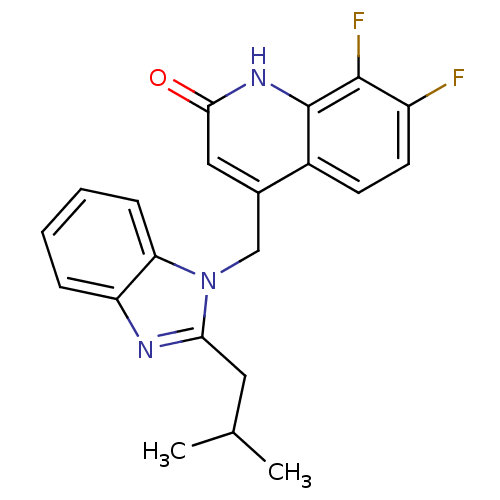
(CHEMBL1801490)Show SMILES CC(C)Cc1nc2ccccc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C21H19F2N3O/c1-12(2)9-18-24-16-5-3-4-6-17(16)26(18)11-13-10-19(27)25-21-14(13)7-8-15(22)20(21)23/h3-8,10,12H,9,11H2,1-2H3,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348722
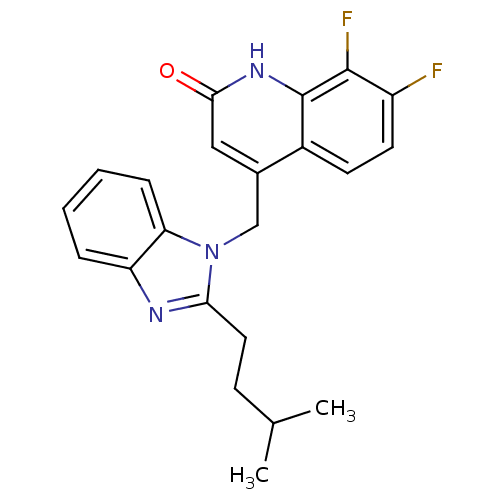
(CHEMBL1801491)Show SMILES CC(C)CCc1nc2ccccc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C22H21F2N3O/c1-13(2)7-10-19-25-17-5-3-4-6-18(17)27(19)12-14-11-20(28)26-22-15(14)8-9-16(23)21(22)24/h3-6,8-9,11,13H,7,10,12H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348718

(CHEMBL1801494)Show SMILES Fc1ccc2c(Cn3c(CC4CCCC4)nc4ccccc34)cc(=O)[nH]c2c1F Show InChI InChI=1S/C23H21F2N3O/c24-17-10-9-16-15(12-21(29)27-23(16)22(17)25)13-28-19-8-4-3-7-18(19)26-20(28)11-14-5-1-2-6-14/h3-4,7-10,12,14H,1-2,5-6,11,13H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348723
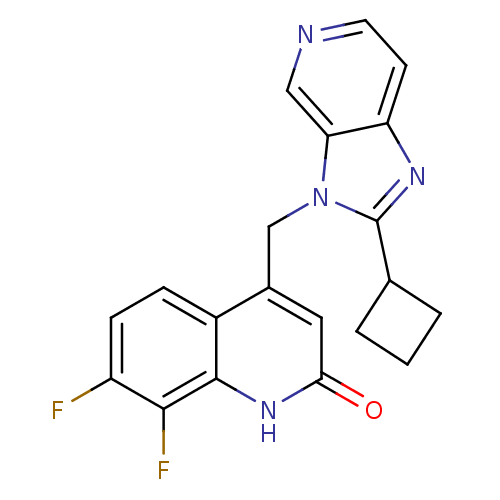
(CHEMBL1801497)Show SMILES Fc1ccc2c(Cn3c(nc4ccncc34)C3CCC3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C20H16F2N4O/c21-14-5-4-13-12(8-17(27)25-19(13)18(14)22)10-26-16-9-23-7-6-15(16)24-20(26)11-2-1-3-11/h4-9,11H,1-3,10H2,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348724
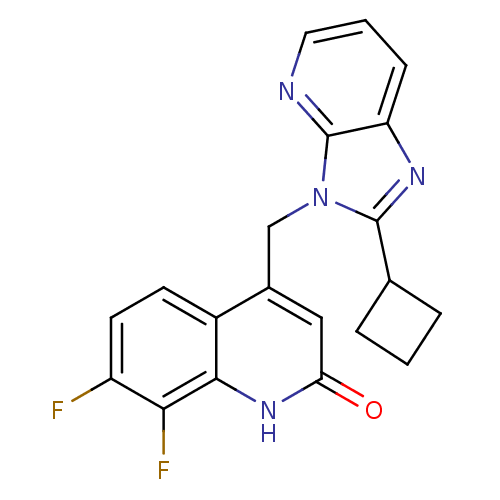
(CHEMBL1801498)Show SMILES Fc1ccc2c(Cn3c(nc4cccnc34)C3CCC3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C20H16F2N4O/c21-14-7-6-13-12(9-16(27)25-18(13)17(14)22)10-26-19(11-3-1-4-11)24-15-5-2-8-23-20(15)26/h2,5-9,11H,1,3-4,10H2,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348725
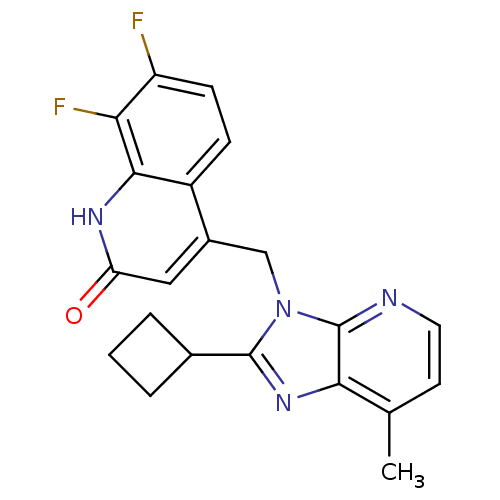
(CHEMBL1801499)Show SMILES Cc1ccnc2n(Cc3cc(=O)[nH]c4c(F)c(F)ccc34)c(nc12)C1CCC1 Show InChI InChI=1S/C21H18F2N4O/c1-11-7-8-24-21-18(11)26-20(12-3-2-4-12)27(21)10-13-9-16(28)25-19-14(13)5-6-15(22)17(19)23/h5-9,12H,2-4,10H2,1H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348726
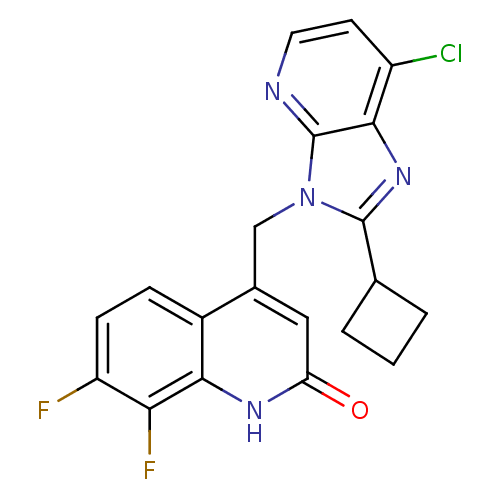
(CHEMBL1801500)Show SMILES Fc1ccc2c(Cn3c(nc4c(Cl)ccnc34)C3CCC3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C20H15ClF2N4O/c21-13-6-7-24-20-18(13)26-19(10-2-1-3-10)27(20)9-11-8-15(28)25-17-12(11)4-5-14(22)16(17)23/h4-8,10H,1-3,9H2,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348727
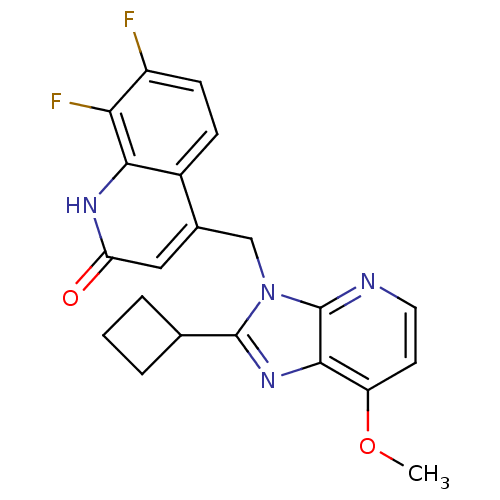
(CHEMBL1801501)Show SMILES COc1ccnc2n(Cc3cc(=O)[nH]c4c(F)c(F)ccc34)c(nc12)C1CCC1 Show InChI InChI=1S/C21H18F2N4O2/c1-29-15-7-8-24-21-19(15)26-20(11-3-2-4-11)27(21)10-12-9-16(28)25-18-13(12)5-6-14(22)17(18)23/h5-9,11H,2-4,10H2,1H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348728
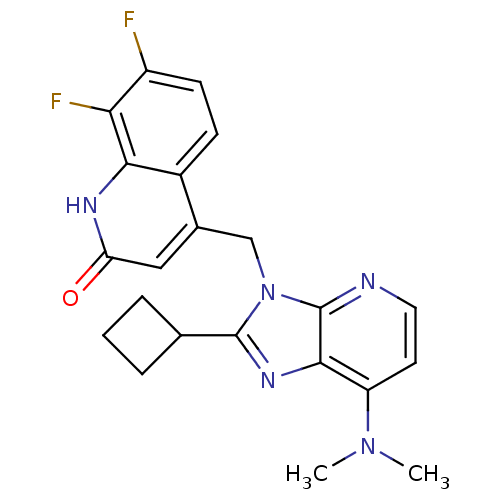
(CHEMBL1801502)Show SMILES CN(C)c1ccnc2n(Cc3cc(=O)[nH]c4c(F)c(F)ccc34)c(nc12)C1CCC1 Show InChI InChI=1S/C22H21F2N5O/c1-28(2)16-8-9-25-22-20(16)27-21(12-4-3-5-12)29(22)11-13-10-17(30)26-19-14(13)6-7-15(23)18(19)24/h6-10,12H,3-5,11H2,1-2H3,(H,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348729
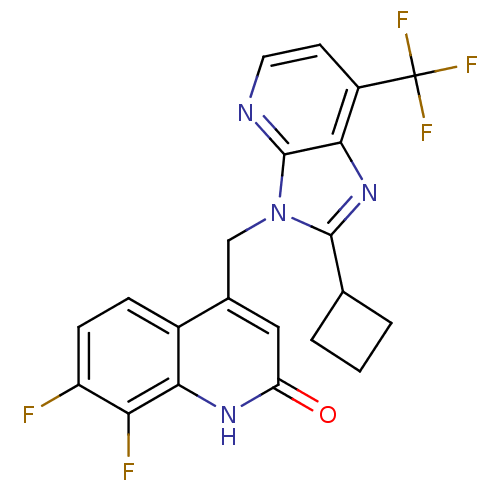
(CHEMBL1801503)Show SMILES Fc1ccc2c(Cn3c(nc4c(ccnc34)C(F)(F)F)C3CCC3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C21H15F5N4O/c22-14-5-4-12-11(8-15(31)28-17(12)16(14)23)9-30-19(10-2-1-3-10)29-18-13(21(24,25)26)6-7-27-20(18)30/h4-8,10H,1-3,9H2,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348730
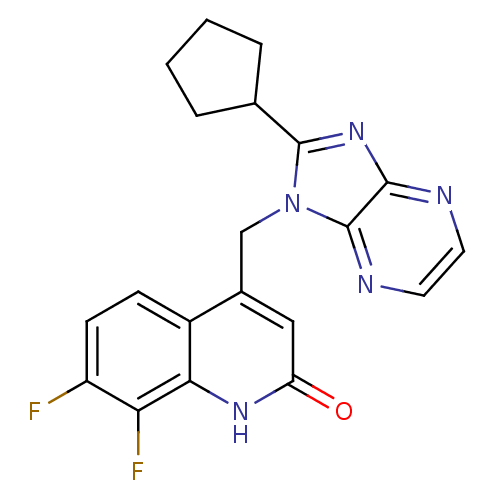
(CHEMBL1801505)Show SMILES Fc1ccc2c(Cn3c(nc4nccnc34)C3CCCC3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C20H17F2N5O/c21-14-6-5-13-12(9-15(28)25-17(13)16(14)22)10-27-19(11-3-1-2-4-11)26-18-20(27)24-8-7-23-18/h5-9,11H,1-4,10H2,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348731
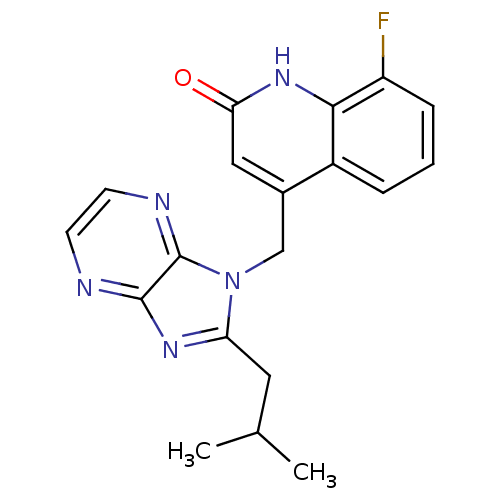
(CHEMBL1801061)Show SMILES CC(C)Cc1nc2nccnc2n1Cc1cc(=O)[nH]c2c(F)cccc12 Show InChI InChI=1S/C19H18FN5O/c1-11(2)8-15-23-18-19(22-7-6-21-18)25(15)10-12-9-16(26)24-17-13(12)4-3-5-14(17)20/h3-7,9,11H,8,10H2,1-2H3,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50348709

(CHEMBL1801062)Show SMILES Fc1cccc2c(Cn3c(nc4nccnc34)C3CC(F)(F)C3)cc(=O)[nH]c12 Show InChI InChI=1S/C19H14F3N5O/c20-13-3-1-2-12-10(6-14(28)25-15(12)13)9-27-17(11-7-19(21,22)8-11)26-16-18(27)24-5-4-23-16/h1-6,11H,7-9H2,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS expressed in HEK293 cells assessed as inhibition A23187 induced nitric oxide production incubated for 24 hrs measured after ... |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50348732
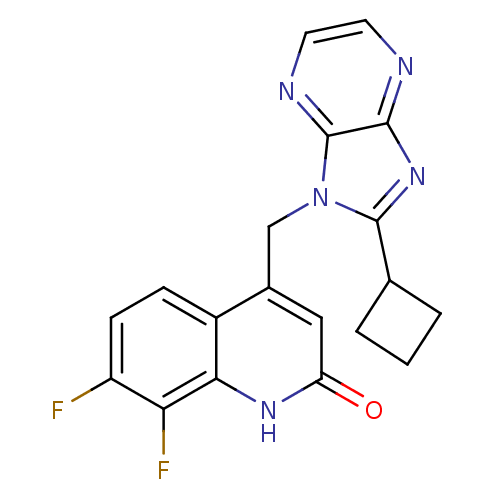
(CHEMBL1801504)Show SMILES Fc1ccc2c(Cn3c(nc4nccnc34)C3CCC3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C19H15F2N5O/c20-13-5-4-12-11(8-14(27)24-16(12)15(13)21)9-26-18(10-2-1-3-10)25-17-19(26)23-7-6-22-17/h4-8,10H,1-3,9H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50348710

(CHEMBL1801506)Show SMILES CC(C)Cc1nc2nccnc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C19H17F2N5O/c1-10(2)7-14-24-18-19(23-6-5-22-18)26(14)9-11-8-15(27)25-17-12(11)3-4-13(20)16(17)21/h3-6,8,10H,7,9H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50348733
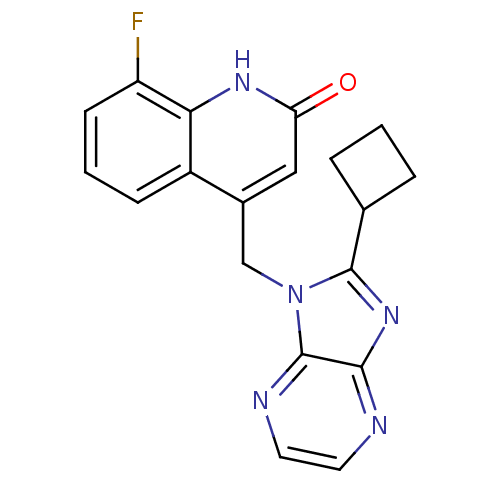
(CHEMBL1801060)Show SMILES Fc1cccc2c(Cn3c(nc4nccnc34)C3CCC3)cc(=O)[nH]c12 Show InChI InChI=1S/C19H16FN5O/c20-14-6-2-5-13-12(9-15(26)23-16(13)14)10-25-18(11-3-1-4-11)24-17-19(25)22-8-7-21-17/h2,5-9,11H,1,3-4,10H2,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50348732

(CHEMBL1801504)Show SMILES Fc1ccc2c(Cn3c(nc4nccnc34)C3CCC3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C19H15F2N5O/c20-13-5-4-12-11(8-14(27)24-16(12)15(13)21)9-26-18(10-2-1-3-10)25-17-19(26)23-7-6-22-17/h4-8,10H,1-3,9H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50348710

(CHEMBL1801506)Show SMILES CC(C)Cc1nc2nccnc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C19H17F2N5O/c1-10(2)7-14-24-18-19(23-6-5-22-18)26(14)9-11-8-15(27)25-17-12(11)3-4-13(20)16(17)21/h3-6,8,10H,7,9H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50348733

(CHEMBL1801060)Show SMILES Fc1cccc2c(Cn3c(nc4nccnc34)C3CCC3)cc(=O)[nH]c12 Show InChI InChI=1S/C19H16FN5O/c20-14-6-2-5-13-12(9-15(26)23-16(13)14)10-25-18(11-3-1-4-11)24-17-19(25)22-8-7-21-17/h2,5-9,11H,1,3-4,10H2,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50348732

(CHEMBL1801504)Show SMILES Fc1ccc2c(Cn3c(nc4nccnc34)C3CCC3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C19H15F2N5O/c20-13-5-4-12-11(8-14(27)24-16(12)15(13)21)9-26-18(10-2-1-3-10)25-17-19(26)23-7-6-22-17/h4-8,10H,1-3,9H2,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50348710

(CHEMBL1801506)Show SMILES CC(C)Cc1nc2nccnc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C19H17F2N5O/c1-10(2)7-14-24-18-19(23-6-5-22-18)26(14)9-11-8-15(27)25-17-12(11)3-4-13(20)16(17)21/h3-6,8,10H,7,9H2,1-2H3,(H,25,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50348733

(CHEMBL1801060)Show SMILES Fc1cccc2c(Cn3c(nc4nccnc34)C3CCC3)cc(=O)[nH]c12 Show InChI InChI=1S/C19H16FN5O/c20-14-6-2-5-13-12(9-15(26)23-16(13)14)10-25-18(11-3-1-4-11)24-17-19(25)22-8-7-21-17/h2,5-9,11H,1,3-4,10H2,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50348709

(CHEMBL1801062)Show SMILES Fc1cccc2c(Cn3c(nc4nccnc34)C3CC(F)(F)C3)cc(=O)[nH]c12 Show InChI InChI=1S/C19H14F3N5O/c20-13-3-1-2-12-10(6-14(28)25-15(12)13)9-27-17(11-7-19(21,22)8-11)26-16-18(27)24-5-4-23-16/h1-6,11H,7-9H2,(H,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50348732

(CHEMBL1801504)Show SMILES Fc1ccc2c(Cn3c(nc4nccnc34)C3CCC3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C19H15F2N5O/c20-13-5-4-12-11(8-14(27)24-16(12)15(13)21)9-26-18(10-2-1-3-10)25-17-19(26)23-7-6-22-17/h4-8,10H,1-3,9H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.80E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50348710

(CHEMBL1801506)Show SMILES CC(C)Cc1nc2nccnc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C19H17F2N5O/c1-10(2)7-14-24-18-19(23-6-5-22-18)26(14)9-11-8-15(27)25-17-12(11)3-4-13(20)16(17)21/h3-6,8,10H,7,9H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50348733

(CHEMBL1801060)Show SMILES Fc1cccc2c(Cn3c(nc4nccnc34)C3CCC3)cc(=O)[nH]c12 Show InChI InChI=1S/C19H16FN5O/c20-14-6-2-5-13-12(9-15(26)23-16(13)14)10-25-18(11-3-1-4-11)24-17-19(25)22-8-7-21-17/h2,5-9,11H,1,3-4,10H2,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50348710

(CHEMBL1801506)Show SMILES CC(C)Cc1nc2nccnc2n1Cc1cc(=O)[nH]c2c(F)c(F)ccc12 Show InChI InChI=1S/C19H17F2N5O/c1-10(2)7-14-24-18-19(23-6-5-22-18)26(14)9-11-8-15(27)25-17-12(11)3-4-13(20)16(17)21/h3-6,8,10H,7,9H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Kalypsys, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 53: 7739-55 (2010)
Article DOI: 10.1021/jm100828n
BindingDB Entry DOI: 10.7270/Q29Z9584 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data