

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II) by esterase method | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124177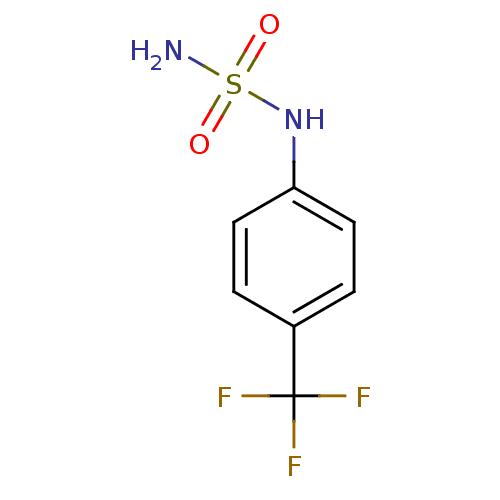 (CHEMBL167179 | N-[4-(trifluoromethyl)phenyl]sulfam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124177 (CHEMBL167179 | N-[4-(trifluoromethyl)phenyl]sulfam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124171 (4-methoxyphenylsulfamide | CHEMBL165878 | N-(4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124175 (CHEMBL353767 | N-(4-hydroxyphenyl)sulfamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124170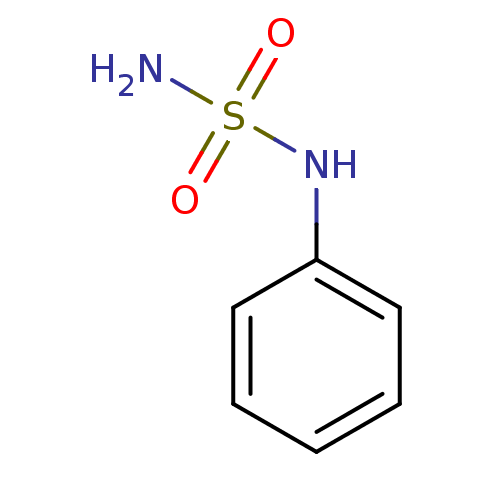 (CHEMBL168514 | N-phenylsulfamide | PHENYLSULFAMIDE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124163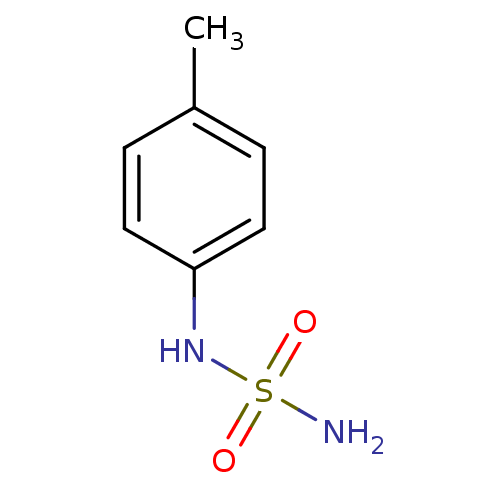 (CHEMBL168511 | N-(4-methylphenyl)sulfamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124170 (CHEMBL168514 | N-phenylsulfamide | PHENYLSULFAMIDE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124157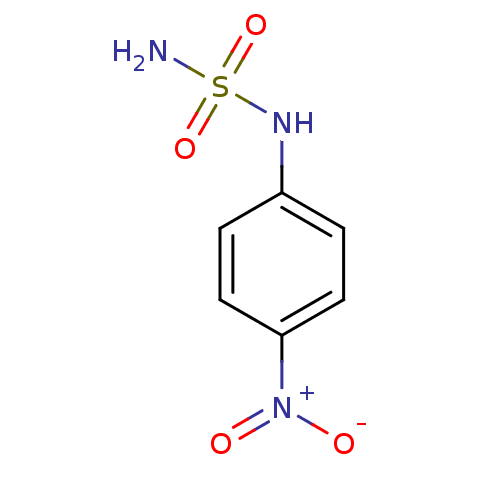 (4-nitrophenylsulfamide | CHEMBL168778 | N-(4-nitro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10881 (CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124171 (4-methoxyphenylsulfamide | CHEMBL165878 | N-(4-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124155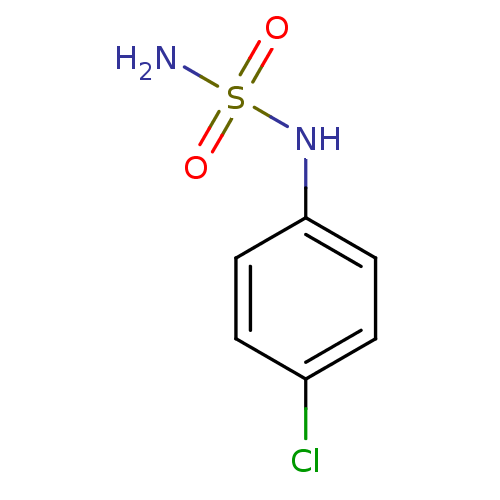 (CHEMBL167048 | N-(4-chlorophenyl)sulfamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124163 (CHEMBL168511 | N-(4-methylphenyl)sulfamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124179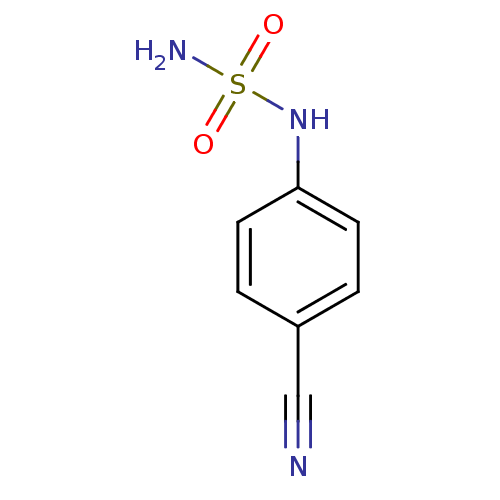 (CHEMBL166340 | N-(4-cyanophenyl)sulfamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124175 (CHEMBL353767 | N-(4-hydroxyphenyl)sulfamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124159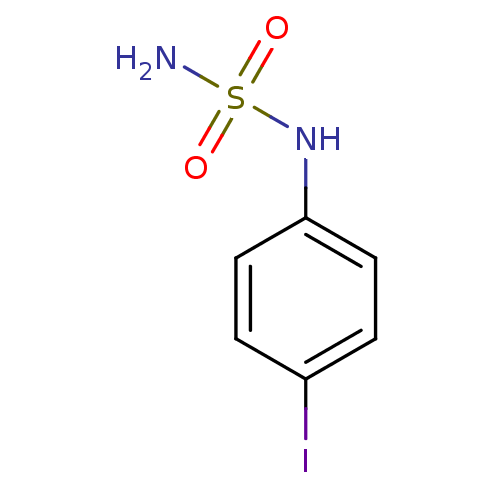 (CHEMBL166446 | N-(4-iodophenyl)sulfamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124162 (CHEMBL354323 | N-[4-(dimethylamino)phenyl]sulfamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124159 (CHEMBL166446 | N-(4-iodophenyl)sulfamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124157 (4-nitrophenylsulfamide | CHEMBL168778 | N-(4-nitro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124155 (CHEMBL167048 | N-(4-chlorophenyl)sulfamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124158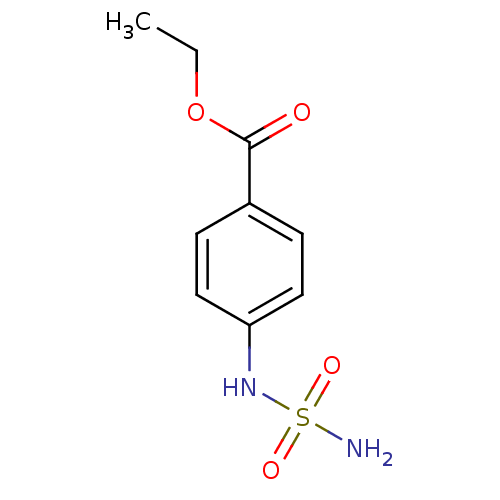 (CHEMBL166708 | ethyl 4-[(aminosulfonyl)amino]benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124179 (CHEMBL166340 | N-(4-cyanophenyl)sulfamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124162 (CHEMBL354323 | N-[4-(dimethylamino)phenyl]sulfamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124178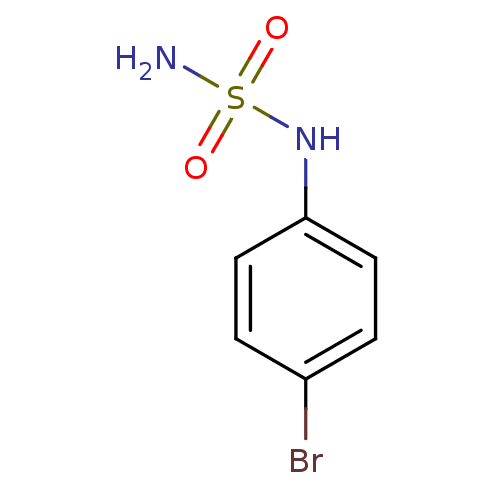 (CHEMBL166686 | N-(4-bromophenyl)sulfamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124178 (CHEMBL166686 | N-(4-bromophenyl)sulfamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124158 (CHEMBL166708 | ethyl 4-[(aminosulfonyl)amino]benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124174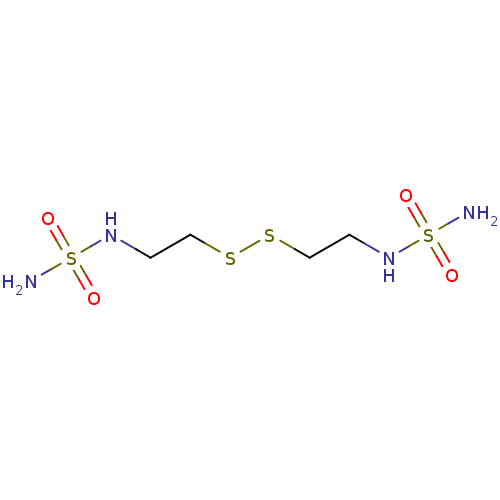 (CHEMBL444608 | N-[2-({2-[(aminosulfonyl)amino]ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124165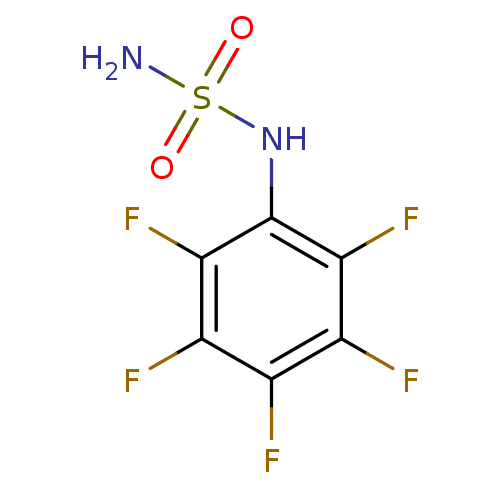 (CHEMBL169486 | N-(pentafluorophenyl)sulfamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124165 (CHEMBL169486 | N-(pentafluorophenyl)sulfamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124167 (CHEMBL168910 | N-2-naphthylsulfamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10883 (4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank MMDB PDB PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124167 (CHEMBL168910 | N-2-naphthylsulfamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124156 (CHEMBL168898 | N-(3-benzoylphenyl)sulfamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124156 (CHEMBL168898 | N-(3-benzoylphenyl)sulfamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124161 (CHEMBL168526 | N-benzylsulfamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124172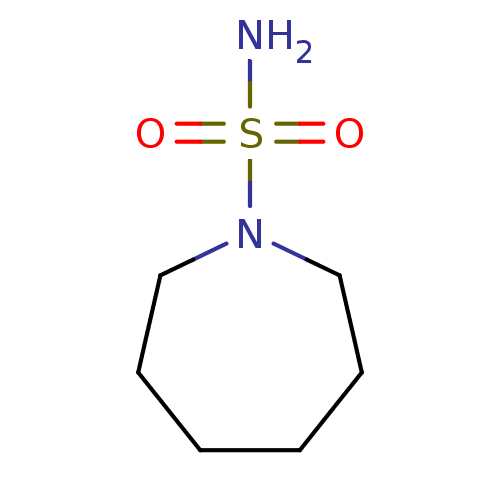 (CHEMBL165939 | azepane-1-sulfonamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124161 (CHEMBL168526 | N-benzylsulfamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124176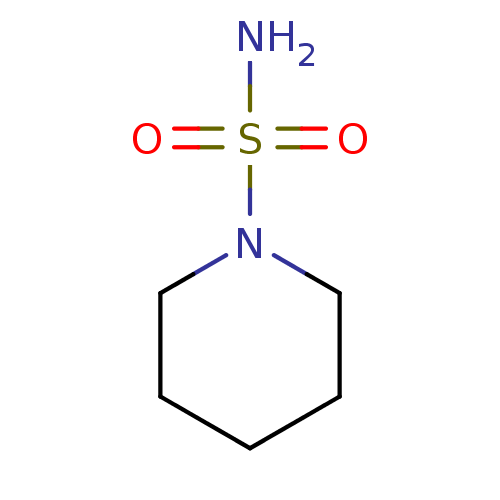 (CHEMBL169370 | piperidine-1-sulfonamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124166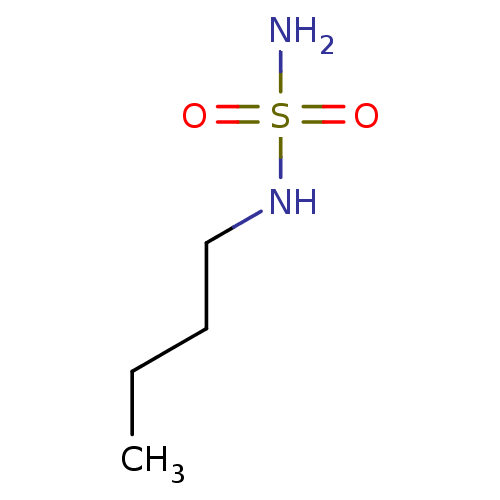 (CHEMBL170015 | N-butylsulfamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124174 (CHEMBL444608 | N-[2-({2-[(aminosulfonyl)amino]ethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124176 (CHEMBL169370 | piperidine-1-sulfonamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124172 (CHEMBL165939 | azepane-1-sulfonamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124160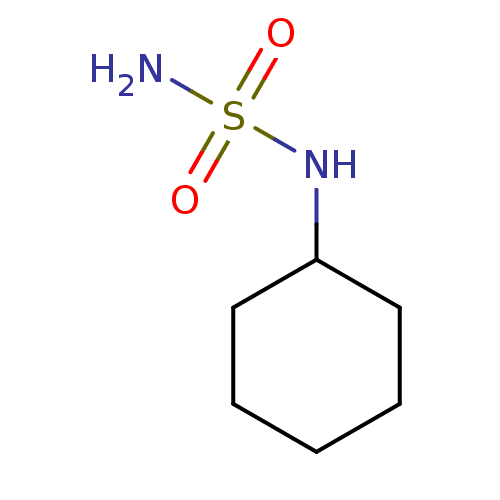 (CHEMBL352592 | N-cyclohexylsulfamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50124166 (CHEMBL170015 | N-butylsulfamide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase I (hCA I), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124160 (CHEMBL352592 | N-cyclohexylsulfamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50124168 (CHEMBL355349 | N,N-dibenzylsulfamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 647 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against human cloned isoenzyme carbonic anhydrase II (hCA II), by esterase method. | Bioorg Med Chem Lett 13: 837-40 (2003) BindingDB Entry DOI: 10.7270/Q2GB24MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 65 total ) | Next | Last >> |