 Found 108 hits Enz. Inhib. hit(s) with all data for entry = 50049306
Found 108 hits Enz. Inhib. hit(s) with all data for entry = 50049306 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238693
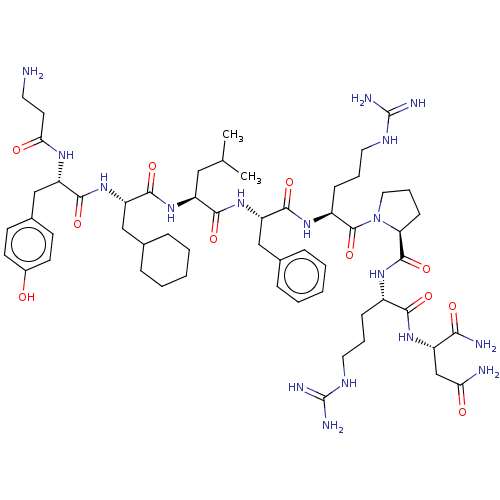
(CHEMBL4063830)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C57H89N17O11/c1-33(2)28-41(71-53(83)44(30-35-14-7-4-8-15-35)73-51(81)42(67-47(77)23-24-58)31-36-19-21-37(75)22-20-36)50(80)72-43(29-34-12-5-3-6-13-34)52(82)69-39(17-10-26-66-57(63)64)55(85)74-27-11-18-45(74)54(84)68-38(16-9-25-65-56(61)62)49(79)70-40(48(60)78)32-46(59)76/h3,5-6,12-13,19-22,33,35,38-45,75H,4,7-11,14-18,23-32,58H2,1-2H3,(H2,59,76)(H2,60,78)(H,67,77)(H,68,84)(H,69,82)(H,70,79)(H,71,83)(H,72,80)(H,73,81)(H4,61,62,65)(H4,63,64,66)/t38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238694

(CHEMBL4098010)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C56H81N17O11/c1-32(2)46(72-51(81)42(29-34-14-7-4-8-15-34)70-49(79)40(66-45(76)23-24-57)30-35-19-21-36(74)22-20-35)53(83)71-41(28-33-12-5-3-6-13-33)50(80)68-38(17-10-26-65-56(62)63)54(84)73-27-11-18-43(73)52(82)67-37(16-9-25-64-55(60)61)48(78)69-39(47(59)77)31-44(58)75/h3-8,12-15,19-22,32,37-43,46,74H,9-11,16-18,23-31,57H2,1-2H3,(H2,58,75)(H2,59,77)(H,66,76)(H,67,82)(H,68,80)(H,69,78)(H,70,79)(H,71,83)(H,72,81)(H4,60,61,64)(H4,62,63,65)/t37-,38-,39-,40-,41-,42-,43-,46-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238695

(CHEMBL4079986)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C54H85N17O11/c1-4-5-14-35(65-48(78)39(27-31(2)3)69-50(80)41(28-32-12-7-6-8-13-32)70-49(79)40(64-44(74)22-23-55)29-33-18-20-34(72)21-19-33)46(76)67-37(16-10-25-63-54(60)61)52(82)71-26-11-17-42(71)51(81)66-36(15-9-24-62-53(58)59)47(77)68-38(45(57)75)30-43(56)73/h6-8,12-13,18-21,31,35-42,72H,4-5,9-11,14-17,22-30,55H2,1-3H3,(H2,56,73)(H2,57,75)(H,64,74)(H,65,78)(H,66,81)(H,67,76)(H,68,77)(H,69,80)(H,70,79)(H4,58,59,62)(H4,60,61,63)/t35-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238696

(CHEMBL4087867)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C56H79N15O12/c1-32(2)27-40(68-53(81)43(29-34-13-7-4-8-14-34)70-51(79)41(64-47(75)23-24-57)30-35-17-19-36(72)20-18-35)50(78)69-42(28-33-11-5-3-6-12-33)52(80)66-38(21-22-45(58)73)55(83)71-26-10-16-44(71)54(82)65-37(15-9-25-63-56(61)62)49(77)67-39(48(60)76)31-46(59)74/h3-8,11-14,17-20,32,37-44,72H,9-10,15-16,21-31,57H2,1-2H3,(H2,58,73)(H2,59,74)(H2,60,76)(H,64,75)(H,65,82)(H,66,80)(H,67,77)(H,68,81)(H,69,78)(H,70,79)(H4,61,62,63)/t37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238688
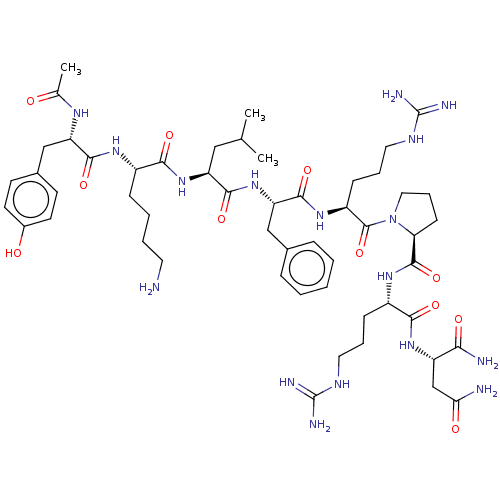
(CHEMBL4082455)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C53H83N17O11/c1-30(2)26-39(68-46(76)35(14-7-8-22-54)64-48(78)40(63-31(3)71)28-33-18-20-34(72)21-19-33)47(77)69-41(27-32-12-5-4-6-13-32)49(79)66-37(16-10-24-62-53(59)60)51(81)70-25-11-17-42(70)50(80)65-36(15-9-23-61-52(57)58)45(75)67-38(44(56)74)29-43(55)73/h4-6,12-13,18-21,30,35-42,72H,7-11,14-17,22-29,54H2,1-3H3,(H2,55,73)(H2,56,74)(H,63,71)(H,64,78)(H,65,80)(H,66,79)(H,67,75)(H,68,76)(H,69,77)(H4,57,58,61)(H4,59,60,62)/t35-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 83 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238697
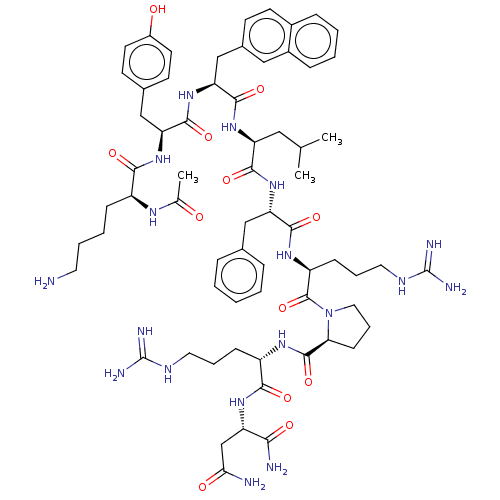
(CHEMBL4090300)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCCN)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C66H94N18O12/c1-38(2)32-50(80-62(94)53(36-42-22-25-43-16-7-8-17-44(43)33-42)83-61(93)52(35-41-23-26-45(86)27-24-41)81-57(89)46(76-39(3)85)18-9-10-28-67)59(91)82-51(34-40-14-5-4-6-15-40)60(92)78-48(20-12-30-75-66(72)73)64(96)84-31-13-21-54(84)63(95)77-47(19-11-29-74-65(70)71)58(90)79-49(56(69)88)37-55(68)87/h4-8,14-17,22-27,33,38,46-54,86H,9-13,18-21,28-32,34-37,67H2,1-3H3,(H2,68,87)(H2,69,88)(H,76,85)(H,77,95)(H,78,92)(H,79,90)(H,80,94)(H,81,89)(H,82,91)(H,83,93)(H4,70,71,74)(H4,72,73,75)/t46-,47-,48-,49-,50-,51-,52-,53-,54-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238698
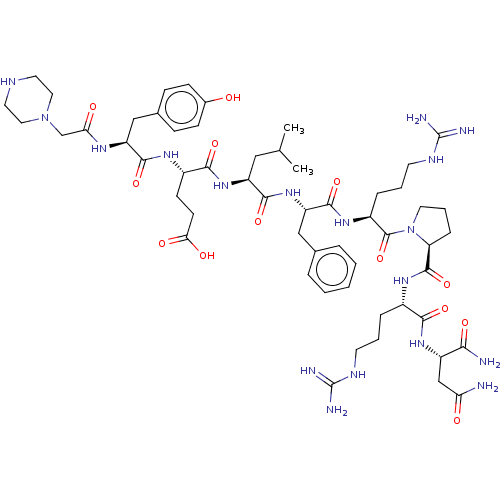
(CHEMBL4069256)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C56H86N18O13/c1-32(2)27-40(71-49(82)37(18-19-46(78)79)67-51(84)41(29-34-14-16-35(75)17-15-34)66-45(77)31-73-25-22-63-23-26-73)50(83)72-42(28-33-9-4-3-5-10-33)52(85)69-38(12-7-21-65-56(61)62)54(87)74-24-8-13-43(74)53(86)68-36(11-6-20-64-55(59)60)48(81)70-39(47(58)80)30-44(57)76/h3-5,9-10,14-17,32,36-43,63,75H,6-8,11-13,18-31H2,1-2H3,(H2,57,76)(H2,58,80)(H,66,77)(H,67,84)(H,68,86)(H,69,85)(H,70,81)(H,71,82)(H,72,83)(H,78,79)(H4,59,60,64)(H4,61,62,65)/t36-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238699

(CHEMBL4063341)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C64H90N18O11/c1-38(2)31-48(78-60(91)51(35-41-18-21-42-13-6-7-14-43(42)32-41)80-58(89)49(34-40-19-22-44(83)23-20-40)74-54(85)37-81-29-26-71-27-30-81)57(88)79-50(33-39-11-4-3-5-12-39)59(90)76-46(16-9-25-73-64(69)70)62(93)82-28-10-17-52(82)61(92)75-45(15-8-24-72-63(67)68)56(87)77-47(55(66)86)36-53(65)84/h3-7,11-14,18-23,32,38,45-52,71,83H,8-10,15-17,24-31,33-37H2,1-2H3,(H2,65,84)(H2,66,86)(H,74,85)(H,75,92)(H,76,90)(H,77,87)(H,78,91)(H,79,88)(H,80,89)(H4,67,68,72)(H4,69,70,73)/t45-,46-,47-,48-,49-,50-,51-,52-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238700

(CHEMBL4089636)Show SMILES CC(C)[C@H](NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C56H87N17O11/c1-32(2)46(72-51(81)42(29-34-14-7-4-8-15-34)70-49(79)40(66-45(76)23-24-57)30-35-19-21-36(74)22-20-35)53(83)71-41(28-33-12-5-3-6-13-33)50(80)68-38(17-10-26-65-56(62)63)54(84)73-27-11-18-43(73)52(82)67-37(16-9-25-64-55(60)61)48(78)69-39(47(59)77)31-44(58)75/h3,5-6,12-13,19-22,32,34,37-43,46,74H,4,7-11,14-18,23-31,57H2,1-2H3,(H2,58,75)(H2,59,77)(H,66,76)(H,67,82)(H,68,80)(H,69,78)(H,70,79)(H,71,83)(H,72,81)(H4,60,61,64)(H4,62,63,65)/t37-,38-,39-,40-,41-,42-,43-,46-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50238688

(CHEMBL4082455)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C53H83N17O11/c1-30(2)26-39(68-46(76)35(14-7-8-22-54)64-48(78)40(63-31(3)71)28-33-18-20-34(72)21-19-33)47(77)69-41(27-32-12-5-4-6-13-32)49(79)66-37(16-10-24-62-53(59)60)51(81)70-25-11-17-42(70)50(80)65-36(15-9-23-61-52(57)58)45(75)67-38(44(56)74)29-43(55)73/h4-6,12-13,18-21,30,35-42,72H,7-11,14-17,22-29,54H2,1-3H3,(H2,55,73)(H2,56,74)(H,63,71)(H,64,78)(H,65,80)(H,66,79)(H,67,75)(H,68,76)(H,69,77)(H4,57,58,61)(H4,59,60,62)/t35-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238689

(CHEMBL4073946)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C55H81N17O11/c1-31(2)26-40(70-53(83)43(28-34-14-8-5-9-15-34)72-51(81)41(66-45(75)22-23-56)29-35-18-20-36(73)21-19-35)50(80)71-42(27-33-12-6-4-7-13-33)52(82)68-37(16-10-24-63-54(59)60)48(78)65-32(3)47(77)67-38(17-11-25-64-55(61)62)49(79)69-39(46(58)76)30-44(57)74/h4-9,12-15,18-21,31-32,37-43,73H,10-11,16-17,22-30,56H2,1-3H3,(H2,57,74)(H2,58,76)(H,65,78)(H,66,75)(H,67,77)(H,68,82)(H,69,79)(H,70,83)(H,71,80)(H,72,81)(H4,59,60,63)(H4,61,62,64)/t32-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50049425
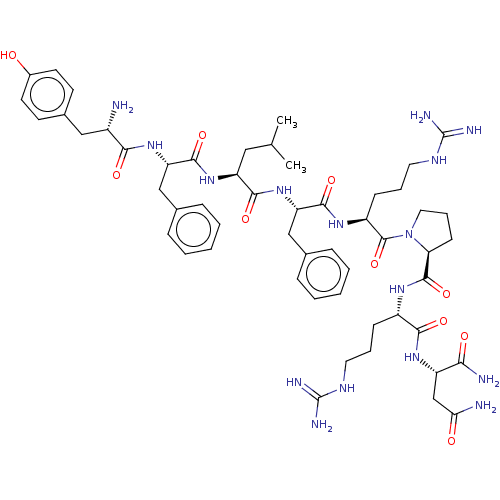
(CHEMBL3356083)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C54H78N16O10/c1-31(2)26-40(68-50(78)41(28-32-12-5-3-6-13-32)67-46(74)36(55)27-34-19-21-35(71)22-20-34)48(76)69-42(29-33-14-7-4-8-15-33)49(77)65-38(17-10-24-63-54(60)61)52(80)70-25-11-18-43(70)51(79)64-37(16-9-23-62-53(58)59)47(75)66-39(45(57)73)30-44(56)72/h3-8,12-15,19-22,31,36-43,71H,9-11,16-18,23-30,55H2,1-2H3,(H2,56,72)(H2,57,73)(H,64,79)(H,65,77)(H,66,75)(H,67,74)(H,68,78)(H,69,76)(H4,58,59,62)(H4,60,61,63)/t36-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238690
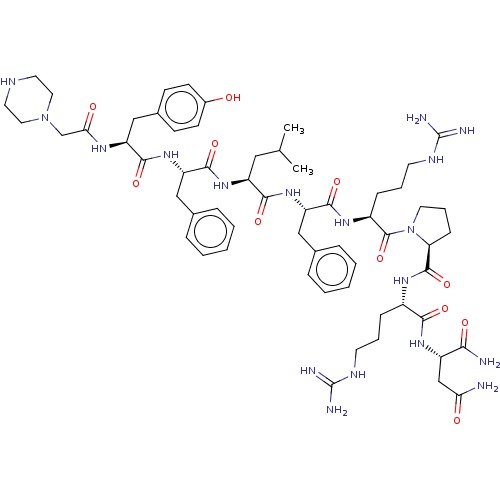
(CHEMBL4068681)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C60H88N18O11/c1-36(2)30-44(74-56(87)47(32-38-14-7-4-8-15-38)76-54(85)45(33-39-19-21-40(79)22-20-39)70-50(81)35-77-28-25-67-26-29-77)53(84)75-46(31-37-12-5-3-6-13-37)55(86)72-42(17-10-24-69-60(65)66)58(89)78-27-11-18-48(78)57(88)71-41(16-9-23-68-59(63)64)52(83)73-43(51(62)82)34-49(61)80/h3-8,12-15,19-22,36,41-48,67,79H,9-11,16-18,23-35H2,1-2H3,(H2,61,80)(H2,62,82)(H,70,81)(H,71,88)(H,72,86)(H,73,83)(H,74,87)(H,75,84)(H,76,85)(H4,63,64,68)(H4,65,66,69)/t41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238691
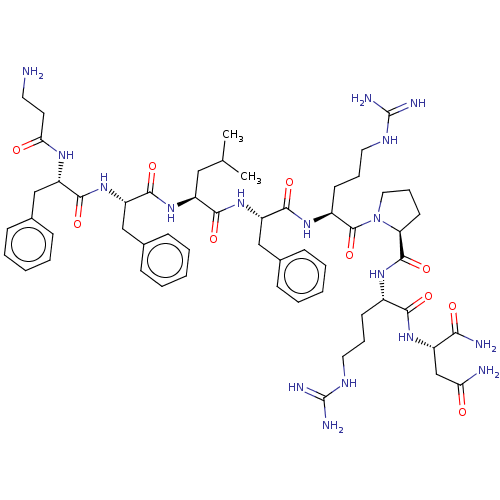
(CHEMBL4088263)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C57H83N17O10/c1-34(2)29-41(71-53(82)44(32-37-19-10-5-11-20-37)73-51(80)42(67-47(76)24-25-58)30-35-15-6-3-7-16-35)50(79)72-43(31-36-17-8-4-9-18-36)52(81)69-39(22-13-27-66-57(63)64)55(84)74-28-14-23-45(74)54(83)68-38(21-12-26-65-56(61)62)49(78)70-40(48(60)77)33-46(59)75/h3-11,15-20,34,38-45H,12-14,21-33,58H2,1-2H3,(H2,59,75)(H2,60,77)(H,67,76)(H,68,83)(H,69,81)(H,70,78)(H,71,82)(H,72,79)(H,73,80)(H4,61,62,65)(H4,63,64,66)/t38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238692
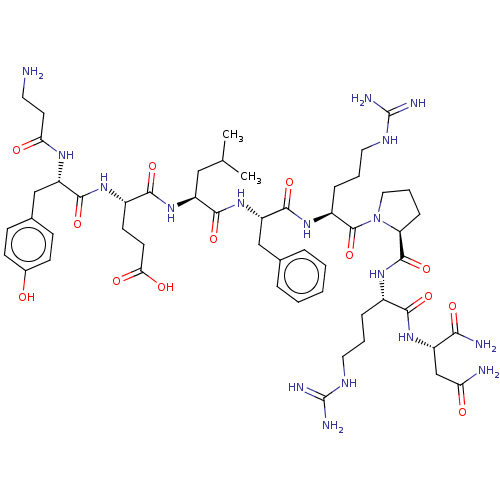
(CHEMBL4060027)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C53H81N17O13/c1-29(2)25-37(68-46(78)34(18-19-43(74)75)64-48(80)38(63-42(73)20-21-54)27-31-14-16-32(71)17-15-31)47(79)69-39(26-30-9-4-3-5-10-30)49(81)66-35(12-7-23-62-53(59)60)51(83)70-24-8-13-40(70)50(82)65-33(11-6-22-61-52(57)58)45(77)67-36(44(56)76)28-41(55)72/h3-5,9-10,14-17,29,33-40,71H,6-8,11-13,18-28,54H2,1-2H3,(H2,55,72)(H2,56,76)(H,63,73)(H,64,80)(H,65,82)(H,66,81)(H,67,77)(H,68,78)(H,69,79)(H,74,75)(H4,57,58,61)(H4,59,60,62)/t33-,34-,35-,36-,37-,38-,39-,40-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50238701

(CHEMBL4061507)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C57H96N18O11/c1-4-5-14-38(49(80)70-40(16-10-23-66-57(62)63)55(86)75-26-11-17-45(75)54(85)69-39(15-9-22-65-56(60)61)50(81)71-41(48(59)79)32-46(58)77)68-51(82)42(29-34(2)3)72-53(84)44(30-35-12-7-6-8-13-35)73-52(83)43(31-36-18-20-37(76)21-19-36)67-47(78)33-74-27-24-64-25-28-74/h18-21,34-35,38-45,64,76H,4-17,22-33H2,1-3H3,(H2,58,77)(H2,59,79)(H,67,78)(H,68,82)(H,69,85)(H,70,80)(H,71,81)(H,72,84)(H,73,83)(H4,60,61,65)(H4,62,63,66)/t38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238704
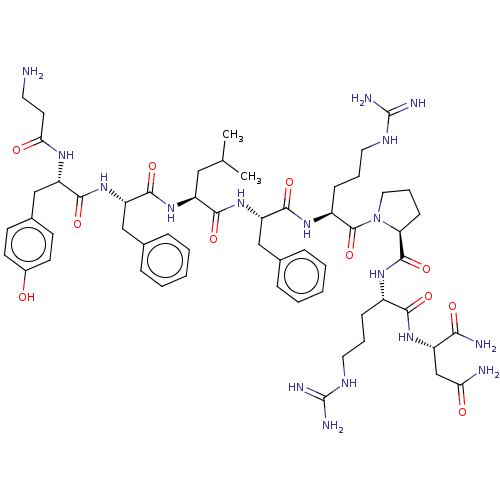
(CHEMBL4095998)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C57H83N17O11/c1-33(2)28-41(71-53(83)44(30-35-14-7-4-8-15-35)73-51(81)42(67-47(77)23-24-58)31-36-19-21-37(75)22-20-36)50(80)72-43(29-34-12-5-3-6-13-34)52(82)69-39(17-10-26-66-57(63)64)55(85)74-27-11-18-45(74)54(84)68-38(16-9-25-65-56(61)62)49(79)70-40(48(60)78)32-46(59)76/h3-8,12-15,19-22,33,38-45,75H,9-11,16-18,23-32,58H2,1-2H3,(H2,59,76)(H2,60,78)(H,67,77)(H,68,84)(H,69,82)(H,70,79)(H,71,83)(H,72,80)(H,73,81)(H4,61,62,65)(H4,63,64,66)/t38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238700

(CHEMBL4089636)Show SMILES CC(C)[C@H](NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C56H87N17O11/c1-32(2)46(72-51(81)42(29-34-14-7-4-8-15-34)70-49(79)40(66-45(76)23-24-57)30-35-19-21-36(74)22-20-35)53(83)71-41(28-33-12-5-3-6-13-33)50(80)68-38(17-10-26-65-56(62)63)54(84)73-27-11-18-43(73)52(82)67-37(16-9-25-64-55(60)61)48(78)69-39(47(59)77)31-44(58)75/h3,5-6,12-13,19-22,32,34,37-43,46,74H,4,7-11,14-18,23-31,57H2,1-2H3,(H2,58,75)(H2,59,77)(H,66,76)(H,67,82)(H,68,80)(H,69,78)(H,70,79)(H,71,83)(H,72,81)(H4,60,61,64)(H4,62,63,65)/t37-,38-,39-,40-,41-,42-,43-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238706
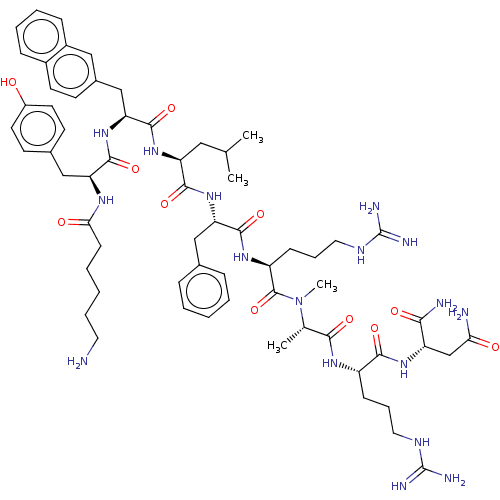
(CHEMBL4099780)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C63H91N17O11/c1-37(2)31-48(77-60(90)51(35-41-22-25-42-17-10-11-18-43(42)32-41)79-58(88)49(34-40-23-26-44(81)27-24-40)73-53(83)21-9-6-12-28-64)57(87)78-50(33-39-15-7-5-8-16-39)59(89)75-46(20-14-30-72-63(69)70)61(91)80(4)38(3)55(85)74-45(19-13-29-71-62(67)68)56(86)76-47(54(66)84)36-52(65)82/h5,7-8,10-11,15-18,22-27,32,37-38,45-51,81H,6,9,12-14,19-21,28-31,33-36,64H2,1-4H3,(H2,65,82)(H2,66,84)(H,73,83)(H,74,85)(H,75,89)(H,76,86)(H,77,90)(H,78,87)(H,79,88)(H4,67,68,71)(H4,69,70,72)/t38-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50238710
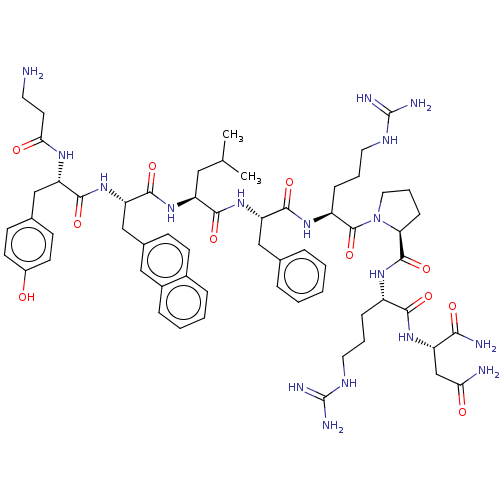
(CHEMBL4091611)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C61H85N17O11/c1-35(2)29-45(75-57(87)48(33-38-18-21-39-13-6-7-14-40(39)30-38)77-55(85)46(71-51(81)24-25-62)32-37-19-22-41(79)23-20-37)54(84)76-47(31-36-11-4-3-5-12-36)56(86)73-43(16-9-27-70-61(67)68)59(89)78-28-10-17-49(78)58(88)72-42(15-8-26-69-60(65)66)53(83)74-44(52(64)82)34-50(63)80/h3-7,11-14,18-23,30,35,42-49,79H,8-10,15-17,24-29,31-34,62H2,1-2H3,(H2,63,80)(H2,64,82)(H,71,81)(H,72,88)(H,73,86)(H,74,83)(H,75,87)(H,76,84)(H,77,85)(H4,65,66,69)(H4,67,68,70)/t42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238708
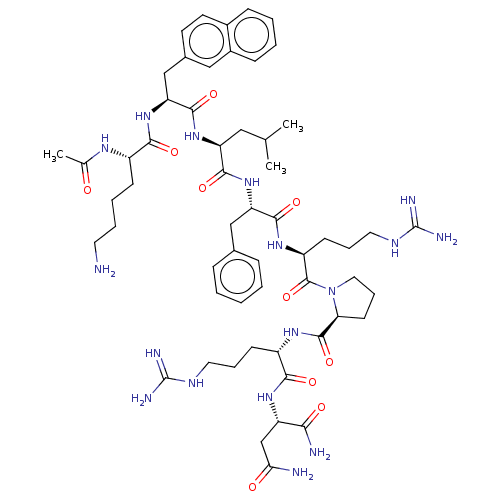
(CHEMBL4060571)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](CCCCN)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C57H85N17O10/c1-33(2)28-43(71-53(82)45(31-36-22-23-37-16-7-8-17-38(37)29-36)72-49(78)39(67-34(3)75)18-9-10-24-58)51(80)73-44(30-35-14-5-4-6-15-35)52(81)69-41(20-12-26-66-57(63)64)55(84)74-27-13-21-46(74)54(83)68-40(19-11-25-65-56(61)62)50(79)70-42(48(60)77)32-47(59)76/h4-8,14-17,22-23,29,33,39-46H,9-13,18-21,24-28,30-32,58H2,1-3H3,(H2,59,76)(H2,60,77)(H,67,75)(H,68,83)(H,69,81)(H,70,79)(H,71,82)(H,72,78)(H,73,80)(H4,61,62,65)(H4,63,64,66)/t39-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50238700

(CHEMBL4089636)Show SMILES CC(C)[C@H](NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C56H87N17O11/c1-32(2)46(72-51(81)42(29-34-14-7-4-8-15-34)70-49(79)40(66-45(76)23-24-57)30-35-19-21-36(74)22-20-35)53(83)71-41(28-33-12-5-3-6-13-33)50(80)68-38(17-10-26-65-56(62)63)54(84)73-27-11-18-43(73)52(82)67-37(16-9-25-64-55(60)61)48(78)69-39(47(59)77)31-44(58)75/h3,5-6,12-13,19-22,32,34,37-43,46,74H,4,7-11,14-18,23-31,57H2,1-2H3,(H2,58,75)(H2,59,77)(H,66,76)(H,67,82)(H,68,80)(H,69,78)(H,70,79)(H,71,83)(H,72,81)(H4,60,61,64)(H4,62,63,65)/t37-,38-,39-,40-,41-,42-,43-,46-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50238709
(CHEMBL4092033)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C55H86N18O13/c1-31(2)26-40(70-49(82)37(18-19-45(77)78)67-51(84)41(28-34-14-16-35(74)17-15-34)65-44(76)30-73-24-22-62-23-25-73)50(83)71-42(27-33-10-6-5-7-11-33)52(85)68-38(13-9-21-64-55(60)61)53(86)72(4)32(3)47(80)66-36(12-8-20-63-54(58)59)48(81)69-39(46(57)79)29-43(56)75/h5-7,10-11,14-17,31-32,36-42,62,74H,8-9,12-13,18-30H2,1-4H3,(H2,56,75)(H2,57,79)(H,65,76)(H,66,80)(H,67,84)(H,68,85)(H,69,81)(H,70,82)(H,71,83)(H,77,78)(H4,58,59,63)(H4,60,61,64)/t32-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50238706

(CHEMBL4099780)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C63H91N17O11/c1-37(2)31-48(77-60(90)51(35-41-22-25-42-17-10-11-18-43(42)32-41)79-58(88)49(34-40-23-26-44(81)27-24-40)73-53(83)21-9-6-12-28-64)57(87)78-50(33-39-15-7-5-8-16-39)59(89)75-46(20-14-30-72-63(69)70)61(91)80(4)38(3)55(85)74-45(19-13-29-71-62(67)68)56(86)76-47(54(66)84)36-52(65)82/h5,7-8,10-11,15-18,22-27,32,37-38,45-51,81H,6,9,12-14,19-21,28-31,33-36,64H2,1-4H3,(H2,65,82)(H2,66,84)(H,73,83)(H,74,85)(H,75,89)(H,76,86)(H,77,90)(H,78,87)(H,79,88)(H4,67,68,71)(H4,69,70,72)/t38-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238701

(CHEMBL4061507)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C57H96N18O11/c1-4-5-14-38(49(80)70-40(16-10-23-66-57(62)63)55(86)75-26-11-17-45(75)54(85)69-39(15-9-22-65-56(60)61)50(81)71-41(48(59)79)32-46(58)77)68-51(82)42(29-34(2)3)72-53(84)44(30-35-12-7-6-8-13-35)73-52(83)43(31-36-18-20-37(76)21-19-36)67-47(78)33-74-27-24-64-25-28-74/h18-21,34-35,38-45,64,76H,4-17,22-33H2,1-3H3,(H2,58,77)(H2,59,79)(H,67,78)(H,68,82)(H,69,85)(H,70,80)(H,71,81)(H,72,84)(H,73,83)(H4,60,61,65)(H4,62,63,66)/t38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50238708

(CHEMBL4060571)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](CCCCN)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C57H85N17O10/c1-33(2)28-43(71-53(82)45(31-36-22-23-37-16-7-8-17-38(37)29-36)72-49(78)39(67-34(3)75)18-9-10-24-58)51(80)73-44(30-35-14-5-4-6-15-35)52(81)69-41(20-12-26-66-57(63)64)55(84)74-27-13-21-46(74)54(83)68-40(19-11-25-65-56(61)62)50(79)70-42(48(60)77)32-47(59)76/h4-8,14-17,22-23,29,33,39-46H,9-13,18-21,24-28,30-32,58H2,1-3H3,(H2,59,76)(H2,60,77)(H,67,75)(H,68,83)(H,69,81)(H,70,79)(H,71,82)(H,72,78)(H,73,80)(H4,61,62,65)(H4,63,64,66)/t39-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50238704

(CHEMBL4095998)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C57H83N17O11/c1-33(2)28-41(71-53(83)44(30-35-14-7-4-8-15-35)73-51(81)42(67-47(77)23-24-58)31-36-19-21-37(75)22-20-36)50(80)72-43(29-34-12-5-3-6-13-34)52(82)69-39(17-10-26-66-57(63)64)55(85)74-27-11-18-45(74)54(84)68-38(16-9-25-65-56(61)62)49(79)70-40(48(60)78)32-46(59)76/h3-8,12-15,19-22,33,38-45,75H,9-11,16-18,23-32,58H2,1-2H3,(H2,59,76)(H2,60,78)(H,67,77)(H,68,84)(H,69,82)(H,70,79)(H,71,83)(H,72,80)(H,73,81)(H4,61,62,65)(H4,63,64,66)/t38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50238711
(CHEMBL4097355)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccncc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C56H88N18O11/c1-32(2)27-40(71-52(83)42(28-33-9-4-3-5-10-33)73-50(81)41(67-46(77)18-21-57)29-34-14-16-36(75)17-15-34)49(80)72-43(30-35-19-24-64-25-20-35)51(82)69-38(12-7-23-66-56(62)63)54(85)74-26-8-13-44(74)53(84)68-37(11-6-22-65-55(60)61)48(79)70-39(47(59)78)31-45(58)76/h14-17,19-20,24-25,32-33,37-44,75H,3-13,18,21-23,26-31,57H2,1-2H3,(H2,58,76)(H2,59,78)(H,67,77)(H,68,84)(H,69,82)(H,70,79)(H,71,83)(H,72,80)(H,73,81)(H4,60,61,65)(H4,62,63,66)/t37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50238691

(CHEMBL4088263)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C57H83N17O10/c1-34(2)29-41(71-53(82)44(32-37-19-10-5-11-20-37)73-51(80)42(67-47(76)24-25-58)30-35-15-6-3-7-16-35)50(79)72-43(31-36-17-8-4-9-18-36)52(81)69-39(22-13-27-66-57(63)64)55(84)74-28-14-23-45(74)54(83)68-38(21-12-26-65-56(61)62)49(78)70-40(48(60)77)33-46(59)75/h3-11,15-20,34,38-45H,12-14,21-33,58H2,1-2H3,(H2,59,75)(H2,60,77)(H,67,76)(H,68,83)(H,69,81)(H,70,78)(H,71,82)(H,72,79)(H,73,80)(H4,61,62,65)(H4,63,64,66)/t38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Mus musculus) | BDBM50238702
(CHEMBL4071218)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C60H81N15O12/c1-34(2)28-44(72-57(85)47(32-37-16-19-38-12-6-7-13-39(38)29-37)74-55(83)45(68-51(79)24-25-61)31-36-17-20-40(76)21-18-36)54(82)73-46(30-35-10-4-3-5-11-35)56(84)70-42(22-23-49(62)77)59(87)75-27-9-15-48(75)58(86)69-41(14-8-26-67-60(65)66)53(81)71-43(52(64)80)33-50(63)78/h3-7,10-13,16-21,29,34,41-48,76H,8-9,14-15,22-28,30-33,61H2,1-2H3,(H2,62,77)(H2,63,78)(H2,64,80)(H,68,79)(H,69,86)(H,70,84)(H,71,81)(H,72,85)(H,73,82)(H,74,83)(H4,65,66,67)/t41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50238702

(CHEMBL4071218)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C60H81N15O12/c1-34(2)28-44(72-57(85)47(32-37-16-19-38-12-6-7-13-39(38)29-37)74-55(83)45(68-51(79)24-25-61)31-36-17-20-40(76)21-18-36)54(82)73-46(30-35-10-4-3-5-11-35)56(84)70-42(22-23-49(62)77)59(87)75-27-9-15-48(75)58(86)69-41(14-8-26-67-60(65)66)53(81)71-43(52(64)80)33-50(63)78/h3-7,10-13,16-21,29,34,41-48,76H,8-9,14-15,22-28,30-33,61H2,1-2H3,(H2,62,77)(H2,63,78)(H2,64,80)(H,68,79)(H,69,86)(H,70,84)(H,71,81)(H,72,85)(H,73,82)(H,74,83)(H4,65,66,67)/t41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238691

(CHEMBL4088263)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C57H83N17O10/c1-34(2)29-41(71-53(82)44(32-37-19-10-5-11-20-37)73-51(80)42(67-47(76)24-25-58)30-35-15-6-3-7-16-35)50(79)72-43(31-36-17-8-4-9-18-36)52(81)69-39(22-13-27-66-57(63)64)55(84)74-28-14-23-45(74)54(83)68-38(21-12-26-65-56(61)62)49(78)70-40(48(60)77)33-46(59)75/h3-11,15-20,34,38-45H,12-14,21-33,58H2,1-2H3,(H2,59,75)(H2,60,77)(H,67,76)(H,68,83)(H,69,81)(H,70,78)(H,71,82)(H,72,79)(H,73,80)(H4,61,62,65)(H4,63,64,66)/t38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50238699

(CHEMBL4063341)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C64H90N18O11/c1-38(2)31-48(78-60(91)51(35-41-18-21-42-13-6-7-14-43(42)32-41)80-58(89)49(34-40-19-22-44(83)23-20-40)74-54(85)37-81-29-26-71-27-30-81)57(88)79-50(33-39-11-4-3-5-12-39)59(90)76-46(16-9-25-73-64(69)70)62(93)82-28-10-17-52(82)61(92)75-45(15-8-24-72-63(67)68)56(87)77-47(55(66)86)36-53(65)84/h3-7,11-14,18-23,32,38,45-52,71,83H,8-10,15-17,24-31,33-37H2,1-2H3,(H2,65,84)(H2,66,86)(H,74,85)(H,75,92)(H,76,90)(H,77,87)(H,78,91)(H,79,88)(H,80,89)(H4,67,68,72)(H4,69,70,73)/t45-,46-,47-,48-,49-,50-,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238705

(CHEMBL4091208)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C52H79N17O13/c1-28(2)42(68-45(77)33(18-19-41(73)74)63-46(78)36(62-40(72)20-21-53)26-30-14-16-31(70)17-15-30)49(81)67-37(25-29-9-4-3-5-10-29)47(79)65-34(12-7-23-61-52(58)59)50(82)69-24-8-13-38(69)48(80)64-32(11-6-22-60-51(56)57)44(76)66-35(43(55)75)27-39(54)71/h3-5,9-10,14-17,28,32-38,42,70H,6-8,11-13,18-27,53H2,1-2H3,(H2,54,71)(H2,55,75)(H,62,72)(H,63,78)(H,64,80)(H,65,79)(H,66,76)(H,67,81)(H,68,77)(H,73,74)(H4,56,57,60)(H4,58,59,61)/t32-,33-,34-,35-,36-,37-,38-,42-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238693

(CHEMBL4063830)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C57H89N17O11/c1-33(2)28-41(71-53(83)44(30-35-14-7-4-8-15-35)73-51(81)42(67-47(77)23-24-58)31-36-19-21-37(75)22-20-36)50(80)72-43(29-34-12-5-3-6-13-34)52(82)69-39(17-10-26-66-57(63)64)55(85)74-27-11-18-45(74)54(84)68-38(16-9-25-65-56(61)62)49(79)70-40(48(60)78)32-46(59)76/h3,5-6,12-13,19-22,33,35,38-45,75H,4,7-11,14-18,23-32,58H2,1-2H3,(H2,59,76)(H2,60,78)(H,67,77)(H,68,84)(H,69,82)(H,70,79)(H,71,83)(H,72,80)(H,73,81)(H4,61,62,65)(H4,63,64,66)/t38-,39-,40-,41-,42-,43-,44-,45-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238696

(CHEMBL4087867)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C56H79N15O12/c1-32(2)27-40(68-53(81)43(29-34-13-7-4-8-14-34)70-51(79)41(64-47(75)23-24-57)30-35-17-19-36(72)20-18-35)50(78)69-42(28-33-11-5-3-6-12-33)52(80)66-38(21-22-45(58)73)55(83)71-26-10-16-44(71)54(82)65-37(15-9-25-63-56(61)62)49(77)67-39(48(60)76)31-46(59)74/h3-8,11-14,17-20,32,37-44,72H,9-10,15-16,21-31,57H2,1-2H3,(H2,58,73)(H2,59,74)(H2,60,76)(H,64,75)(H,65,82)(H,66,80)(H,67,77)(H,68,81)(H,69,78)(H,70,79)(H4,61,62,63)/t37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50238688

(CHEMBL4082455)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C53H83N17O11/c1-30(2)26-39(68-46(76)35(14-7-8-22-54)64-48(78)40(63-31(3)71)28-33-18-20-34(72)21-19-33)47(77)69-41(27-32-12-5-4-6-13-32)49(79)66-37(16-10-24-62-53(59)60)51(81)70-25-11-17-42(70)50(80)65-36(15-9-23-61-52(57)58)45(75)67-38(44(56)74)29-43(55)73/h4-6,12-13,18-21,30,35-42,72H,7-11,14-17,22-29,54H2,1-3H3,(H2,55,73)(H2,56,74)(H,63,71)(H,64,78)(H,65,80)(H,66,79)(H,67,75)(H,68,76)(H,69,77)(H4,57,58,61)(H4,59,60,62)/t35-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238688

(CHEMBL4082455)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C53H83N17O11/c1-30(2)26-39(68-46(76)35(14-7-8-22-54)64-48(78)40(63-31(3)71)28-33-18-20-34(72)21-19-33)47(77)69-41(27-32-12-5-4-6-13-32)49(79)66-37(16-10-24-62-53(59)60)51(81)70-25-11-17-42(70)50(80)65-36(15-9-23-61-52(57)58)45(75)67-38(44(56)74)29-43(55)73/h4-6,12-13,18-21,30,35-42,72H,7-11,14-17,22-29,54H2,1-3H3,(H2,55,73)(H2,56,74)(H,63,71)(H,64,78)(H,65,80)(H,66,79)(H,67,75)(H,68,76)(H,69,77)(H4,57,58,61)(H4,59,60,62)/t35-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50238696

(CHEMBL4087867)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C56H79N15O12/c1-32(2)27-40(68-53(81)43(29-34-13-7-4-8-14-34)70-51(79)41(64-47(75)23-24-57)30-35-17-19-36(72)20-18-35)50(78)69-42(28-33-11-5-3-6-12-33)52(80)66-38(21-22-45(58)73)55(83)71-26-10-16-44(71)54(82)65-37(15-9-25-63-56(61)62)49(77)67-39(48(60)76)31-46(59)74/h3-8,11-14,17-20,32,37-44,72H,9-10,15-16,21-31,57H2,1-2H3,(H2,58,73)(H2,59,74)(H2,60,76)(H,64,75)(H,65,82)(H,66,80)(H,67,77)(H,68,81)(H,69,78)(H,70,79)(H4,61,62,63)/t37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238703
(CHEMBL4081760)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C63H90N18O11/c1-37(2)30-48(77-60(91)51(34-41-18-21-42-14-8-9-15-43(42)31-41)79-58(89)49(33-40-19-22-44(82)23-20-40)73-53(84)36-81-28-26-70-27-29-81)57(88)78-50(32-39-12-6-5-7-13-39)59(90)75-46(17-11-25-72-63(68)69)61(92)80(4)38(3)55(86)74-45(16-10-24-71-62(66)67)56(87)76-47(54(65)85)35-52(64)83/h5-9,12-15,18-23,31,37-38,45-51,70,82H,10-11,16-17,24-30,32-36H2,1-4H3,(H2,64,83)(H2,65,85)(H,73,84)(H,74,86)(H,75,90)(H,76,87)(H,77,91)(H,78,88)(H,79,89)(H4,66,67,71)(H4,68,69,72)/t38-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238692

(CHEMBL4060027)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C53H81N17O13/c1-29(2)25-37(68-46(78)34(18-19-43(74)75)64-48(80)38(63-42(73)20-21-54)27-31-14-16-32(71)17-15-31)47(79)69-39(26-30-9-4-3-5-10-30)49(81)66-35(12-7-23-62-53(59)60)51(83)70-24-8-13-40(70)50(82)65-33(11-6-22-61-52(57)58)45(77)67-36(44(56)76)28-41(55)72/h3-5,9-10,14-17,29,33-40,71H,6-8,11-13,18-28,54H2,1-2H3,(H2,55,72)(H2,56,76)(H,63,73)(H,64,80)(H,65,82)(H,66,81)(H,67,77)(H,68,78)(H,69,79)(H,74,75)(H4,57,58,61)(H4,59,60,62)/t33-,34-,35-,36-,37-,38-,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50238692

(CHEMBL4060027)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C53H81N17O13/c1-29(2)25-37(68-46(78)34(18-19-43(74)75)64-48(80)38(63-42(73)20-21-54)27-31-14-16-32(71)17-15-31)47(79)69-39(26-30-9-4-3-5-10-30)49(81)66-35(12-7-23-62-53(59)60)51(83)70-24-8-13-40(70)50(82)65-33(11-6-22-61-52(57)58)45(77)67-36(44(56)76)28-41(55)72/h3-5,9-10,14-17,29,33-40,71H,6-8,11-13,18-28,54H2,1-2H3,(H2,55,72)(H2,56,76)(H,63,73)(H,64,80)(H,65,82)(H,66,81)(H,67,77)(H,68,78)(H,69,79)(H,74,75)(H4,57,58,61)(H4,59,60,62)/t33-,34-,35-,36-,37-,38-,39-,40-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50238712

(CHEMBL4100718)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)COCC(=O)NCCCOCCOCCOCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C71H106N18O16/c1-44(2)36-55(65(97)87-57(38-46-14-6-5-7-15-46)67(99)84-53(19-11-28-81-71(77)78)69(101)89(4)45(3)63(95)83-52(18-10-27-80-70(75)76)64(96)85-54(62(74)94)41-59(73)91)86-68(100)58(40-48-20-23-49-16-8-9-17-50(49)37-48)88-66(98)56(39-47-21-24-51(90)25-22-47)82-61(93)43-105-42-60(92)79-29-13-31-103-33-35-104-34-32-102-30-12-26-72/h5-9,14-17,20-25,37,44-45,52-58,90H,10-13,18-19,26-36,38-43,72H2,1-4H3,(H2,73,91)(H2,74,94)(H,79,92)(H,82,93)(H,83,95)(H,84,99)(H,85,96)(H,86,100)(H,87,97)(H,88,98)(H4,75,76,80)(H4,77,78,81)/t45-,52-,53-,54-,55-,56-,57-,58-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50238703

(CHEMBL4081760)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C63H90N18O11/c1-37(2)30-48(77-60(91)51(34-41-18-21-42-14-8-9-15-43(42)31-41)79-58(89)49(33-40-19-22-44(82)23-20-40)73-53(84)36-81-28-26-70-27-29-81)57(88)78-50(32-39-12-6-5-7-13-39)59(90)75-46(17-11-25-72-63(68)69)61(92)80(4)38(3)55(86)74-45(16-10-24-71-62(66)67)56(87)76-47(54(65)85)35-52(64)83/h5-9,12-15,18-23,31,37-38,45-51,70,82H,10-11,16-17,24-30,32-36H2,1-4H3,(H2,64,83)(H2,65,85)(H,73,84)(H,74,86)(H,75,90)(H,76,87)(H,77,91)(H,78,88)(H,79,89)(H4,66,67,71)(H4,68,69,72)/t38-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238690

(CHEMBL4068681)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C60H88N18O11/c1-36(2)30-44(74-56(87)47(32-38-14-7-4-8-15-38)76-54(85)45(33-39-19-21-40(79)22-20-39)70-50(81)35-77-28-25-67-26-29-77)53(84)75-46(31-37-12-5-3-6-13-37)55(86)72-42(17-10-24-69-60(65)66)58(89)78-27-11-18-48(78)57(88)71-41(16-9-23-68-59(63)64)52(83)73-43(51(62)82)34-49(61)80/h3-8,12-15,19-22,36,41-48,67,79H,9-11,16-18,23-35H2,1-2H3,(H2,61,80)(H2,62,82)(H,70,81)(H,71,88)(H,72,86)(H,73,83)(H,74,87)(H,75,84)(H,76,85)(H4,63,64,68)(H4,65,66,69)/t41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50238699

(CHEMBL4063341)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CN1CCNCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C64H90N18O11/c1-38(2)31-48(78-60(91)51(35-41-18-21-42-13-6-7-14-43(42)32-41)80-58(89)49(34-40-19-22-44(83)23-20-40)74-54(85)37-81-29-26-71-27-30-81)57(88)79-50(33-39-11-4-3-5-12-39)59(90)76-46(16-9-25-73-64(69)70)62(93)82-28-10-17-52(82)61(92)75-45(15-8-24-72-63(67)68)56(87)77-47(55(66)86)36-53(65)84/h3-7,11-14,18-23,32,38,45-52,71,83H,8-10,15-17,24-31,33-37H2,1-2H3,(H2,65,84)(H2,66,86)(H,74,85)(H,75,92)(H,76,90)(H,77,87)(H,78,91)(H,79,88)(H,80,89)(H4,67,68,72)(H4,69,70,73)/t45-,46-,47-,48-,49-,50-,51-,52-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 1
(Homo sapiens (Human)) | BDBM50049425

(CHEMBL3356083)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C54H78N16O10/c1-31(2)26-40(68-50(78)41(28-32-12-5-3-6-13-32)67-46(74)36(55)27-34-19-21-35(71)22-20-34)48(76)69-42(29-33-14-7-4-8-15-33)49(77)65-38(17-10-24-63-54(60)61)52(80)70-25-11-18-43(70)51(79)64-37(16-9-23-62-53(58)59)47(75)66-39(45(57)73)30-44(56)72/h3-8,12-15,19-22,31,36-43,71H,9-11,16-18,23-30,55H2,1-2H3,(H2,56,72)(H2,57,73)(H,64,79)(H,65,77)(H,66,75)(H,67,74)(H,68,78)(H,69,76)(H4,58,59,62)(H4,60,61,63)/t36-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR1 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50238689

(CHEMBL4073946)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C55H81N17O11/c1-31(2)26-40(70-53(83)43(28-34-14-8-5-9-15-34)72-51(81)41(66-45(75)22-23-56)29-35-18-20-36(73)21-19-35)50(80)71-42(27-33-12-6-4-7-13-33)52(82)68-37(16-10-24-63-54(59)60)48(78)65-32(3)47(77)67-38(17-11-25-64-55(61)62)49(79)69-39(46(58)76)30-44(57)74/h4-9,12-15,18-21,31-32,37-43,73H,10-11,16-17,22-30,56H2,1-3H3,(H2,57,74)(H2,58,76)(H,65,78)(H,66,75)(H,67,77)(H,68,82)(H,69,79)(H,70,83)(H,71,80)(H,72,81)(H4,59,60,63)(H4,61,62,64)/t32-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Mus musculus) | BDBM50238697

(CHEMBL4090300)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCCN)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C66H94N18O12/c1-38(2)32-50(80-62(94)53(36-42-22-25-43-16-7-8-17-44(43)33-42)83-61(93)52(35-41-23-26-45(86)27-24-41)81-57(89)46(76-39(3)85)18-9-10-28-67)59(91)82-51(34-40-14-5-4-6-15-40)60(92)78-48(20-12-30-75-66(72)73)64(96)84-31-13-21-54(84)63(95)77-47(19-11-29-74-65(70)71)58(90)79-49(56(69)88)37-55(68)87/h4-8,14-17,22-27,33,38,46-54,86H,9-13,18-21,28-32,34-37,67H2,1-3H3,(H2,68,87)(H2,69,88)(H,76,85)(H,77,95)(H,78,92)(H,79,90)(H,80,94)(H,81,89)(H,82,91)(H,83,93)(H4,70,71,74)(H4,72,73,75)/t46-,47-,48-,49-,50-,51-,52-,53-,54-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at mouse NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |
Neuromedin-U receptor 2
(Homo sapiens (Human)) | BDBM50238694

(CHEMBL4098010)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| Show InChI InChI=1S/C56H81N17O11/c1-32(2)46(72-51(81)42(29-34-14-7-4-8-15-34)70-49(79)40(66-45(76)23-24-57)30-35-19-21-36(74)22-20-35)53(83)71-41(28-33-12-5-3-6-13-33)50(80)68-38(17-10-26-65-56(62)63)54(84)73-27-11-18-43(73)52(82)67-37(16-9-25-64-55(60)61)48(78)69-39(47(59)77)31-44(58)75/h3-8,12-15,19-22,32,37-43,46,74H,9-11,16-18,23-31,57H2,1-2H3,(H2,58,75)(H2,59,77)(H,66,76)(H,67,82)(H,68,80)(H,69,78)(H,70,79)(H,71,83)(H,72,81)(H4,60,61,64)(H4,62,63,65)/t37-,38-,39-,40-,41-,42-,43-,46-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human NMUR2 expressed in CHO cells assessed as induction of Ca2+ flux measured for 180 secs by Fluo 4-AM dye-based FLIPR assay |
J Med Chem 60: 6089-6097 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00330
BindingDB Entry DOI: 10.7270/Q2W37ZKK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































