

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50102738 (CHEMBL1204162 | CHEMBL281799 | [2-(1-Benzyl-piperi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of acetylcholinesterase from human erythrocytes | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50102726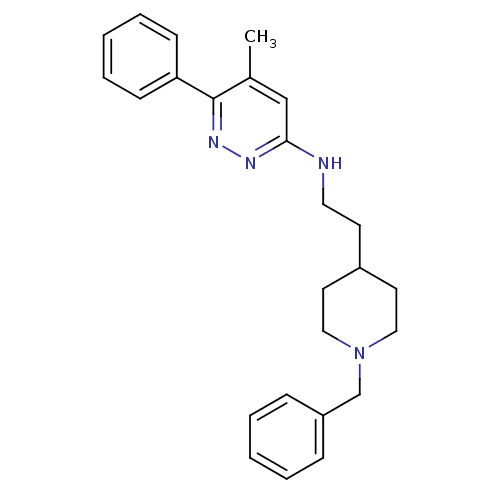 (CHEMBL1204299 | CHEMBL91240 | N-(2-(1-benzylpiperi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase from human serum | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369842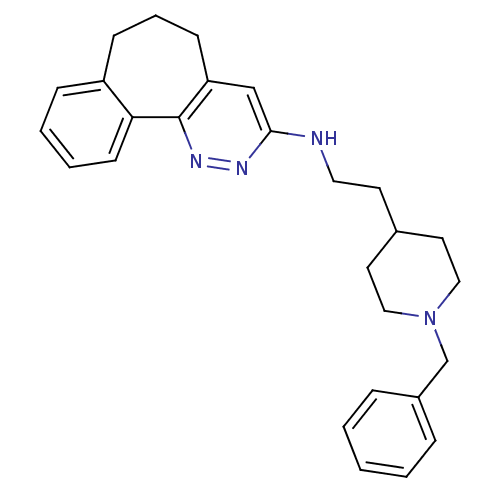 (CHEMBL1202865) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369848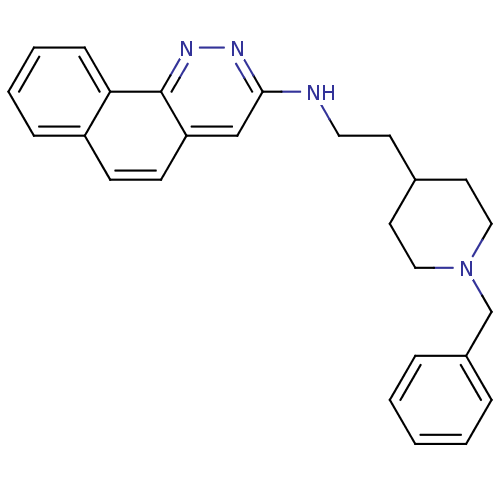 (CHEMBL1202884) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50102729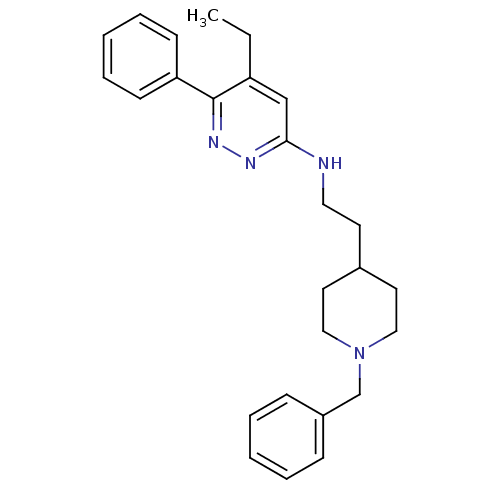 (CHEMBL1204296 | CHEMBL90264 | N-(2-(1-benzylpiperi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369837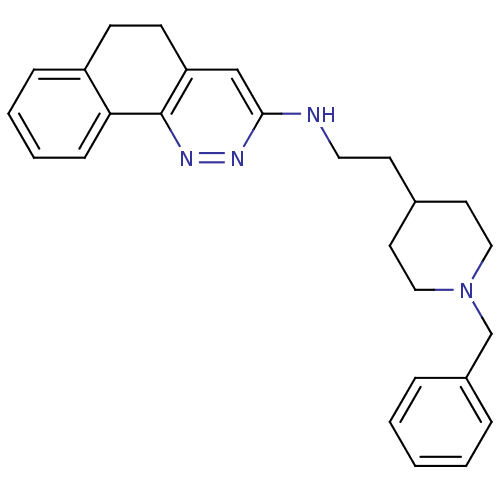 (CHEMBL1202885) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50102726 (CHEMBL1204299 | CHEMBL91240 | N-(2-(1-benzylpiperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of acetylcholinesterase from human erythrocytes | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369849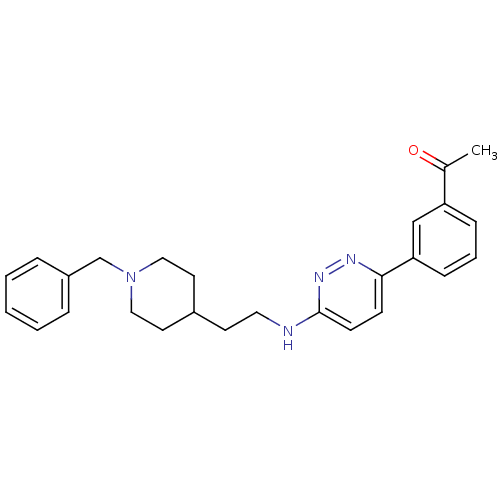 (CHEMBL1202882) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369868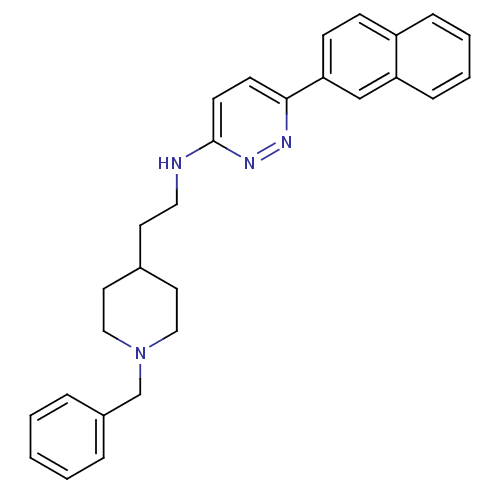 (CHEMBL1202859) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369858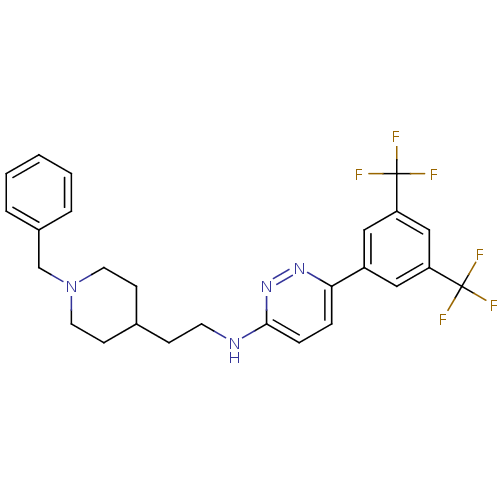 (CHEMBL1202860) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369853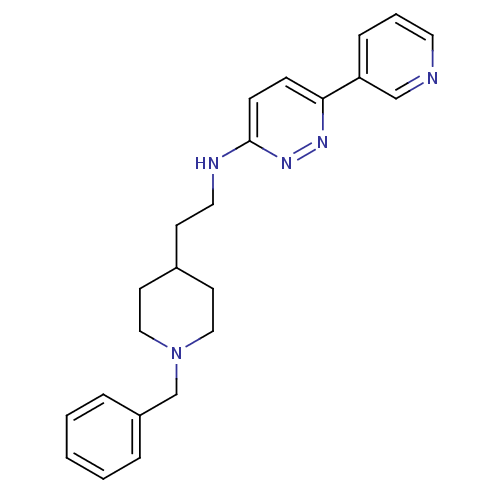 (CHEMBL1202877) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369867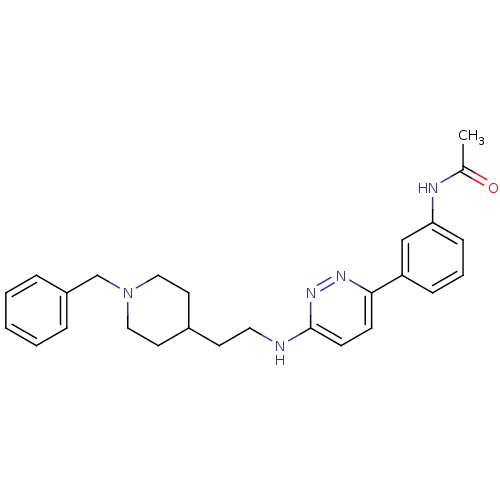 (CHEMBL1202883) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50102743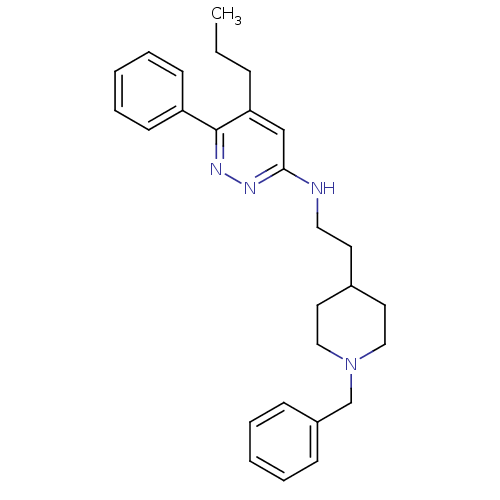 (CHEMBL1204295 | CHEMBL91432 | N-(2-(1-benzylpiperi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50102729 (CHEMBL1204296 | CHEMBL90264 | N-(2-(1-benzylpiperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of acetylcholinesterase from human erythrocytes | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50102735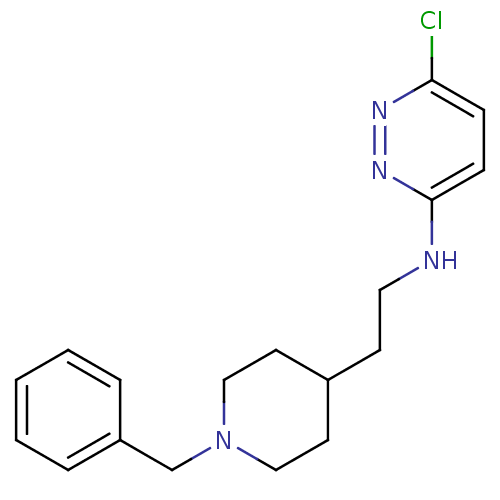 (CHEMBL91538 | [2-(1-Benzyl-piperidin-4-yl)-ethyl]-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369839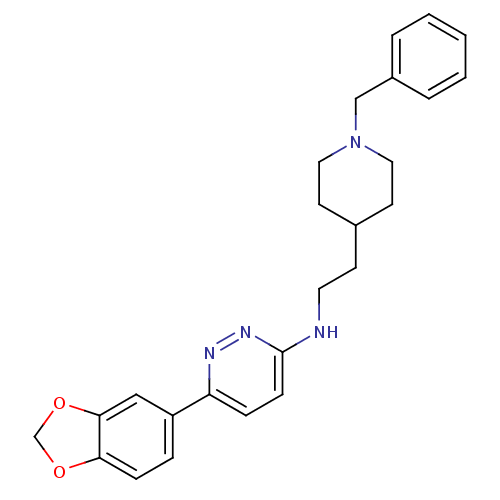 (CHEMBL1202881) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50102738 (CHEMBL1204162 | CHEMBL281799 | [2-(1-Benzyl-piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of acetylcholinesterase from human erythrocytes | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50102755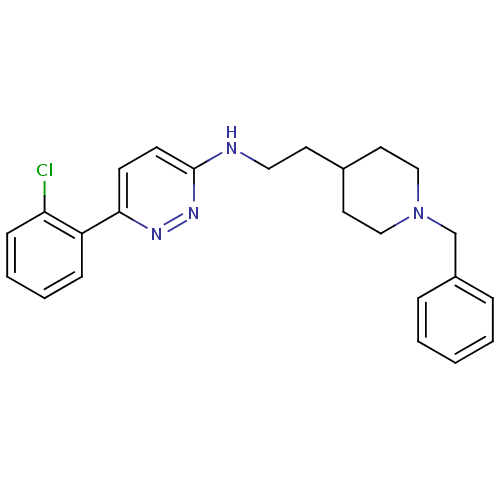 (CHEMBL313415 | [2-(1-Benzyl-piperidin-4-yl)-ethyl]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369866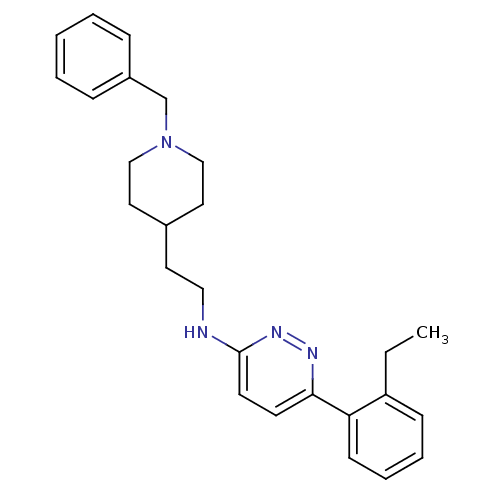 (CHEMBL1202864) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369844 (CHEMBL1202868) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of acetylcholinesterase from human erythrocytes | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369843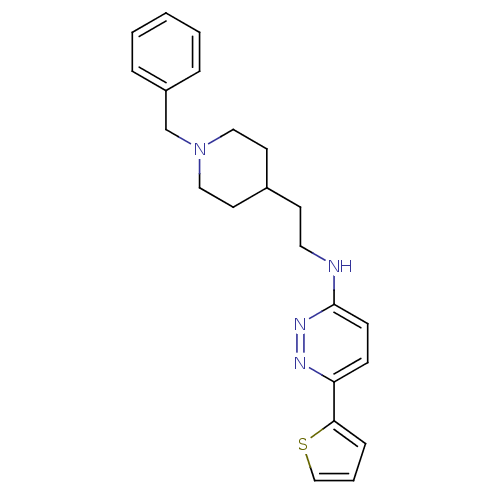 (CHEMBL1202878) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369851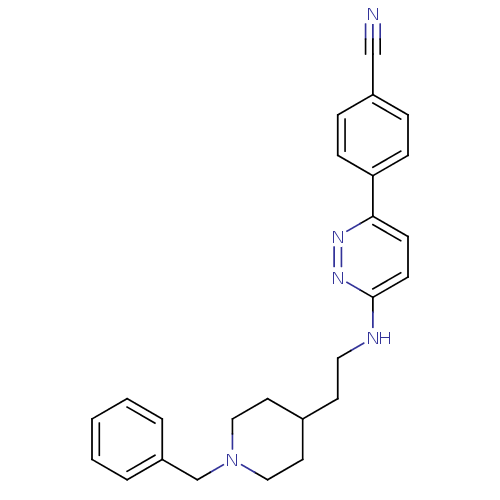 (CHEMBL1202880) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369854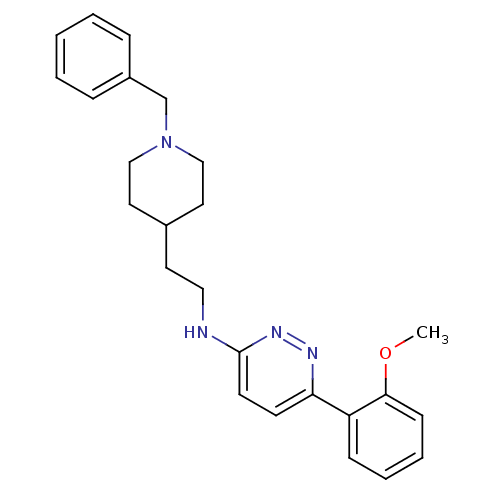 (CHEMBL1202866) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50369842 (CHEMBL1202865) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of acetylcholinesterase from human erythrocytes | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50074277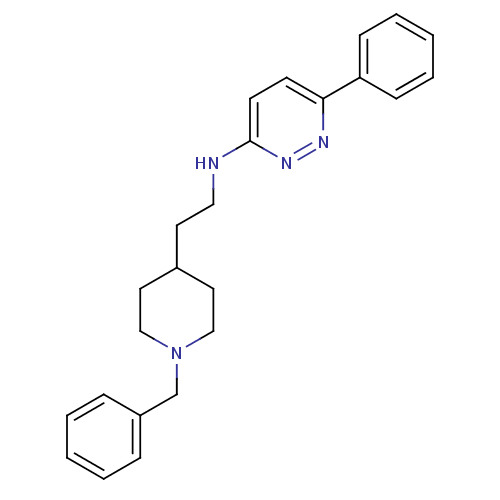 (CHEMBL25412 | [2-(1-Benzyl-piperidin-4-yl)-ethyl]-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50074277 (CHEMBL25412 | [2-(1-Benzyl-piperidin-4-yl)-ethyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of acetylcholinesterase from human erythrocytes | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50369837 (CHEMBL1202885) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of acetylcholinesterase from human erythrocytes | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50369848 (CHEMBL1202884) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of acetylcholinesterase from human erythrocytes | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369865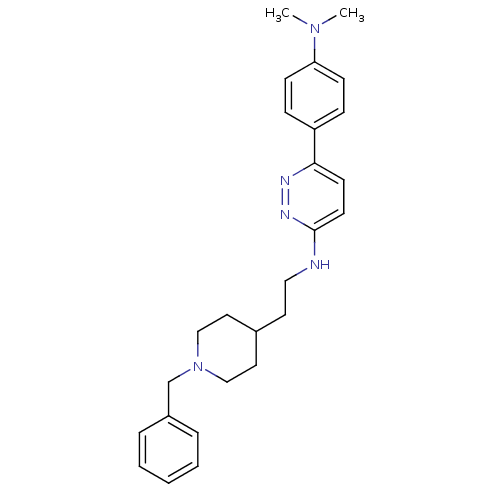 (CHEMBL1202879) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369855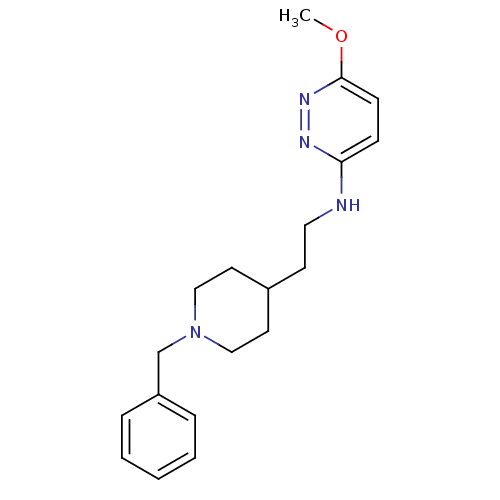 (CHEMBL1202869) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50102738 (CHEMBL1204162 | CHEMBL281799 | [2-(1-Benzyl-piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase from human serum | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369850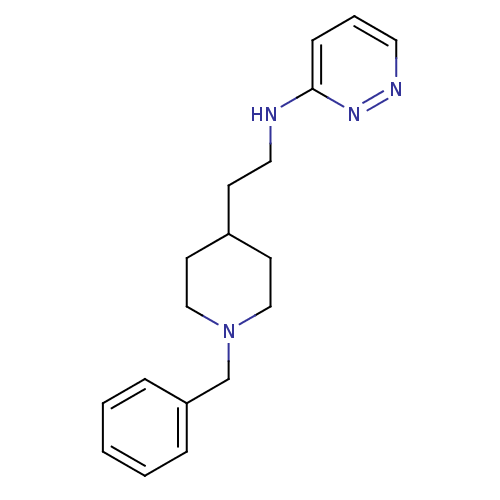 (CHEMBL1202870) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50369848 (CHEMBL1202884) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase from human serum | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50369837 (CHEMBL1202885) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase from human serum | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50102754 (CHEMBL1204300 | CHEMBL94054 | N-(2-(1-benzylpiperi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50369860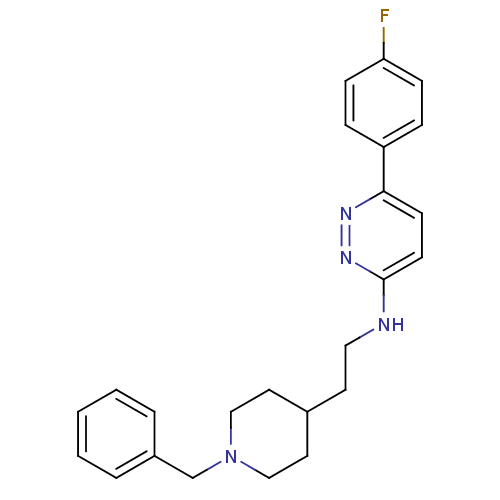 (CHEMBL1202876) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50102757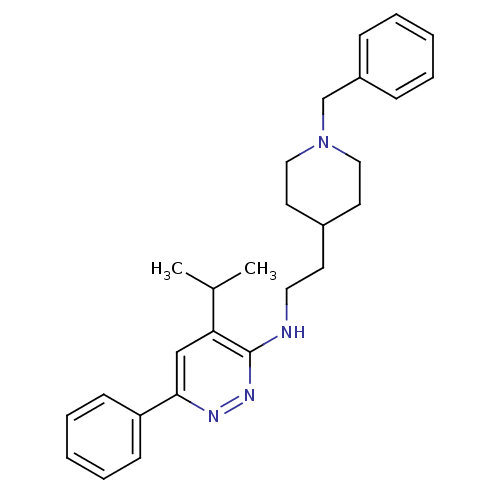 (CHEMBL1204298 | CHEMBL90241 | N-(2-(1-benzylpiperi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50102737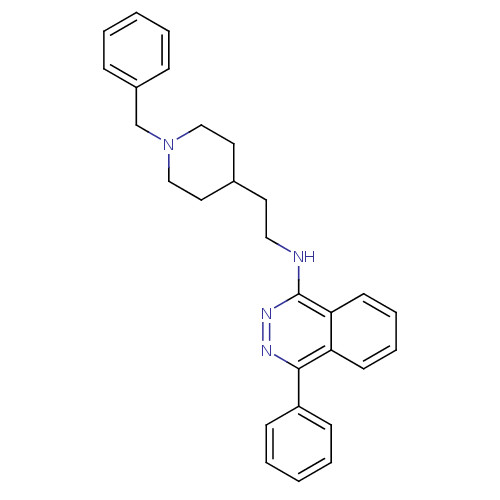 (CHEMBL1204297 | CHEMBL91774 | N-(2-(1-benzylpiperi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of electric eel acetylcholinesterase | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50369861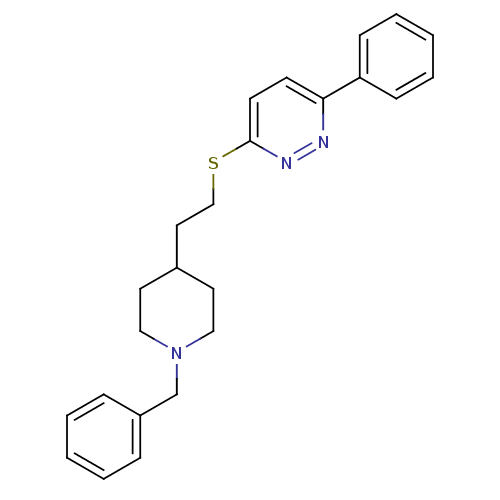 (CHEMBL1202874) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of acetylcholinesterase from human erythrocytes | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50369861 (CHEMBL1202874) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase from human serum | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50369842 (CHEMBL1202865) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase from human serum | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50074277 (CHEMBL25412 | [2-(1-Benzyl-piperidin-4-yl)-ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase from human serum | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50369840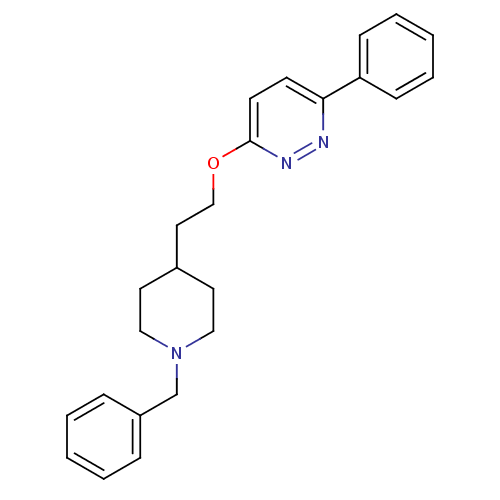 (CHEMBL1202875) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description In vitro inhibition of acetylcholinesterase from human erythrocytes | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50102729 (CHEMBL1204296 | CHEMBL90264 | N-(2-(1-benzylpiperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase from human serum | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50102726 (CHEMBL1204299 | CHEMBL91240 | N-(2-(1-benzylpiperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Louis Pasteur Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase from human serum | J Med Chem 44: 2707-18 (2001) BindingDB Entry DOI: 10.7270/Q2222VG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |