 Found 96 hits Enz. Inhib. hit(s) with all data for entry = 50000991
Found 96 hits Enz. Inhib. hit(s) with all data for entry = 50000991 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267130
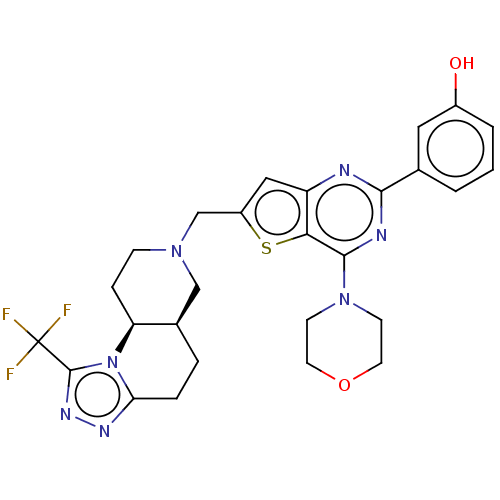
(CHEMBL4096987)Show SMILES [H][C@]12CCc3nnc(n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc(O)c1)C2)C(F)(F)F |r| Show InChI InChI=1S/C27H28F3N7O2S/c28-27(29,30)26-34-33-22-5-4-17-14-35(7-6-21(17)37(22)26)15-19-13-20-23(40-19)25(36-8-10-39-11-9-36)32-24(31-20)16-2-1-3-18(38)12-16/h1-3,12-13,17,21,38H,4-11,14-15H2/t17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267123
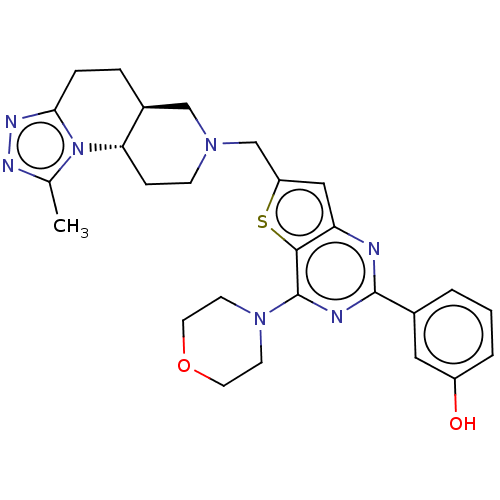
(CHEMBL4094734)Show SMILES [H][C@@]12CCc3nnc(C)n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc(O)c1)C2 |r| Show InChI InChI=1S/C27H31N7O2S/c1-17-30-31-24-6-5-19-15-32(8-7-23(19)34(17)24)16-21-14-22-25(37-21)27(33-9-11-36-12-10-33)29-26(28-22)18-3-2-4-20(35)13-18/h2-4,13-14,19,23,35H,5-12,15-16H2,1H3/t19-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267133
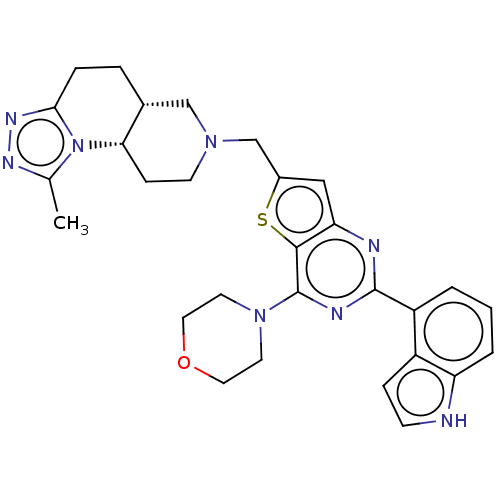
(CHEMBL4081998)Show SMILES [H][C@]12CCc3nnc(C)n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc3[nH]ccc13)C2 |r| Show InChI InChI=1S/C29H32N8OS/c1-18-33-34-26-6-5-19-16-35(10-8-25(19)37(18)26)17-20-15-24-27(39-20)29(36-11-13-38-14-12-36)32-28(31-24)22-3-2-4-23-21(22)7-9-30-23/h2-4,7,9,15,19,25,30H,5-6,8,10-14,16-17H2,1H3/t19-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267132
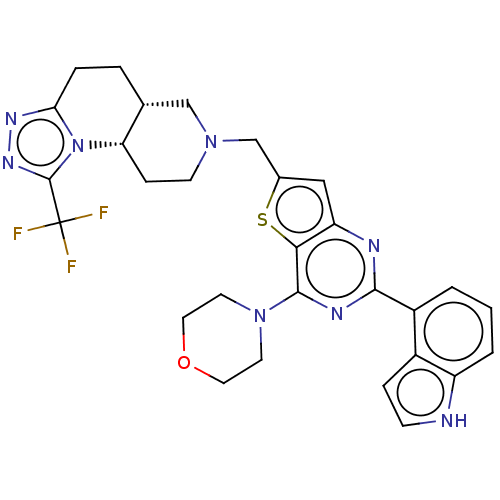
(CHEMBL4068538)Show SMILES [H][C@]12CCc3nnc(n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc3[nH]ccc13)C2)C(F)(F)F |r| Show InChI InChI=1S/C29H29F3N8OS/c30-29(31,32)28-37-36-24-5-4-17-15-38(9-7-23(17)40(24)28)16-18-14-22-25(42-18)27(39-10-12-41-13-11-39)35-26(34-22)20-2-1-3-21-19(20)6-8-33-21/h1-3,6,8,14,17,23,33H,4-5,7,9-13,15-16H2/t17-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267127
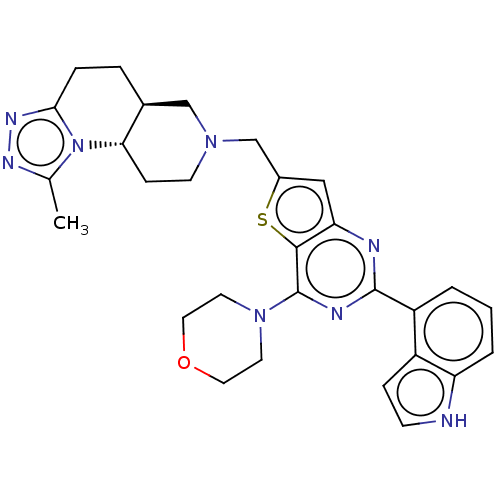
(CHEMBL4061794)Show SMILES [H][C@@]12CCc3nnc(C)n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc3[nH]ccc13)C2 |r| Show InChI InChI=1S/C29H32N8OS/c1-18-33-34-26-6-5-19-16-35(10-8-25(19)37(18)26)17-20-15-24-27(39-20)29(36-11-13-38-14-12-36)32-28(31-24)22-3-2-4-23-21(22)7-9-30-23/h2-4,7,9,15,19,25,30H,5-6,8,10-14,16-17H2,1H3/t19-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267120
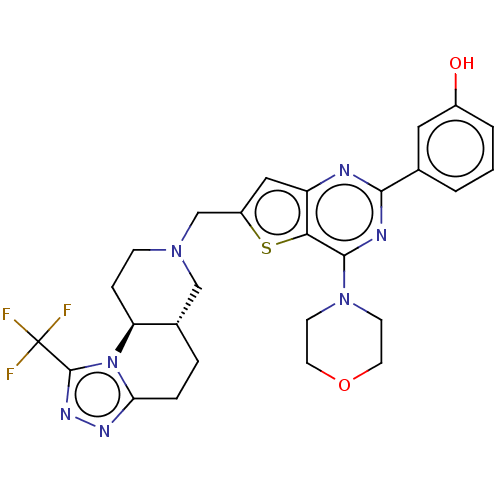
(CHEMBL4071462)Show SMILES [H][C@@]12CCc3nnc(n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc(O)c1)C2)C(F)(F)F |r| Show InChI InChI=1S/C27H28F3N7O2S/c28-27(29,30)26-34-33-22-5-4-17-14-35(7-6-21(17)37(22)26)15-19-13-20-23(40-19)25(36-8-10-39-11-9-36)32-24(31-20)16-2-1-3-18(38)12-16/h1-3,12-13,17,21,38H,4-11,14-15H2/t17-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267122
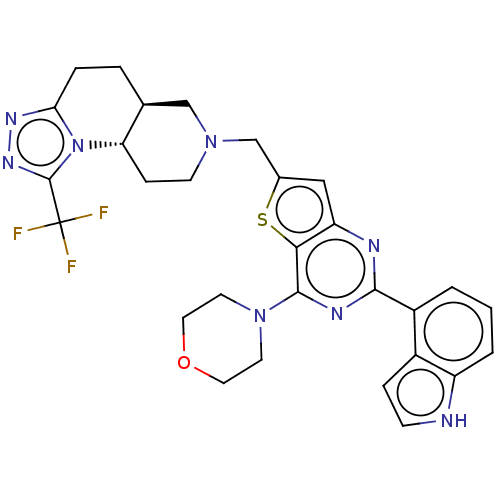
(CHEMBL4097593)Show SMILES [H][C@@]12CCc3nnc(n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc3[nH]ccc13)C2)C(F)(F)F |r| Show InChI InChI=1S/C29H29F3N8OS/c30-29(31,32)28-37-36-24-5-4-17-15-38(9-7-23(17)40(24)28)16-18-14-22-25(42-18)27(39-10-12-41-13-11-39)35-26(34-22)20-2-1-3-21-19(20)6-8-33-21/h1-3,6,8,14,17,23,33H,4-5,7,9-13,15-16H2/t17-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267129
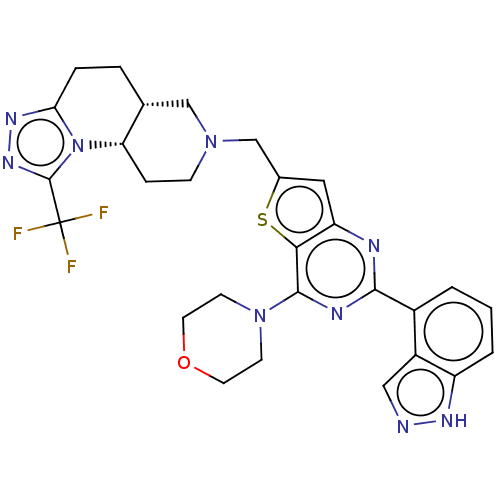
(CHEMBL4078972)Show SMILES [H][C@]12CCc3nnc(n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc3[nH]ncc13)C2)C(F)(F)F |r| Show InChI InChI=1S/C28H28F3N9OS/c29-28(30,31)27-37-36-23-5-4-16-14-38(7-6-22(16)40(23)27)15-17-12-21-24(42-17)26(39-8-10-41-11-9-39)34-25(33-21)18-2-1-3-20-19(18)13-32-35-20/h1-3,12-13,16,22H,4-11,14-15H2,(H,32,35)/t16-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267167
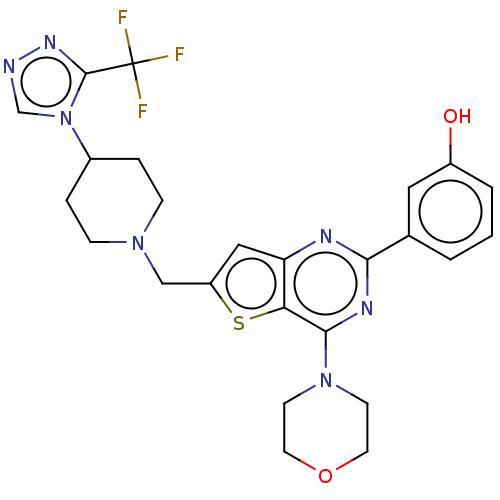
(CHEMBL4081413)Show SMILES Oc1cccc(c1)-c1nc(N2CCOCC2)c2sc(CN3CCC(CC3)n3cnnc3C(F)(F)F)cc2n1 Show InChI InChI=1S/C25H26F3N7O2S/c26-25(27,28)24-32-29-15-35(24)17-4-6-33(7-5-17)14-19-13-20-21(38-19)23(34-8-10-37-11-9-34)31-22(30-20)16-2-1-3-18(36)12-16/h1-3,12-13,15,17,36H,4-11,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267131
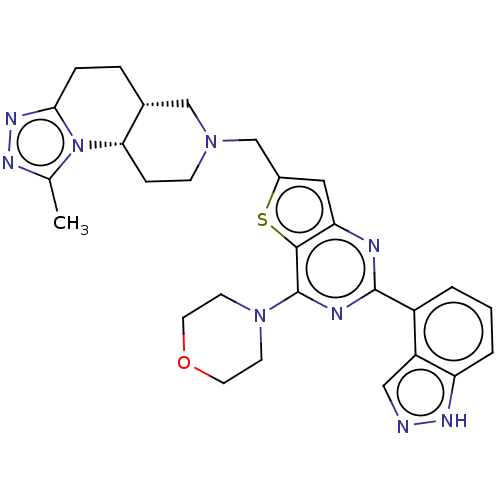
(CHEMBL4100380)Show SMILES [H][C@]12CCc3nnc(C)n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc3[nH]ncc13)C2 |r| Show InChI InChI=1S/C28H31N9OS/c1-17-32-34-25-6-5-18-15-35(8-7-24(18)37(17)25)16-19-13-23-26(39-19)28(36-9-11-38-12-10-36)31-27(30-23)20-3-2-4-22-21(20)14-29-33-22/h2-4,13-14,18,24H,5-12,15-16H2,1H3,(H,29,33)/t18-,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392138
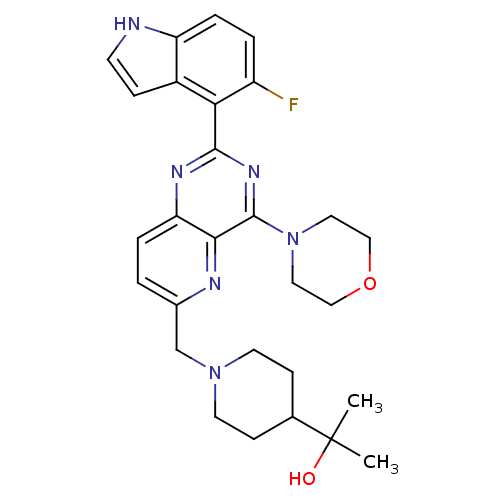
(CHEMBL2152768)Show SMILES CC(C)(O)C1CCN(Cc2ccc3nc(nc(N4CCOCC4)c3n2)-c2c(F)ccc3[nH]ccc23)CC1 Show InChI InChI=1S/C28H33FN6O2/c1-28(2,36)18-8-11-34(12-9-18)17-19-3-5-23-25(31-19)27(35-13-15-37-16-14-35)33-26(32-23)24-20-7-10-30-22(20)6-4-21(24)29/h3-7,10,18,30,36H,8-9,11-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267121
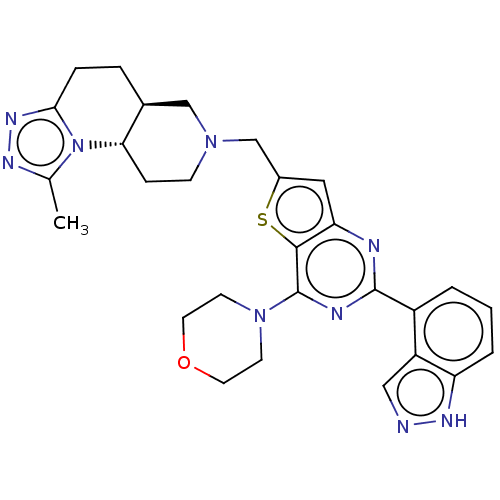
(CHEMBL4070417)Show SMILES [H][C@@]12CCc3nnc(C)n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc3[nH]ncc13)C2 |r| Show InChI InChI=1S/C28H31N9OS/c1-17-32-34-25-6-5-18-15-35(8-7-24(18)37(17)25)16-19-13-23-26(39-19)28(36-9-11-38-12-10-36)31-27(30-23)20-3-2-4-22-21(20)14-29-33-22/h2-4,13-14,18,24H,5-12,15-16H2,1H3,(H,29,33)/t18-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267164
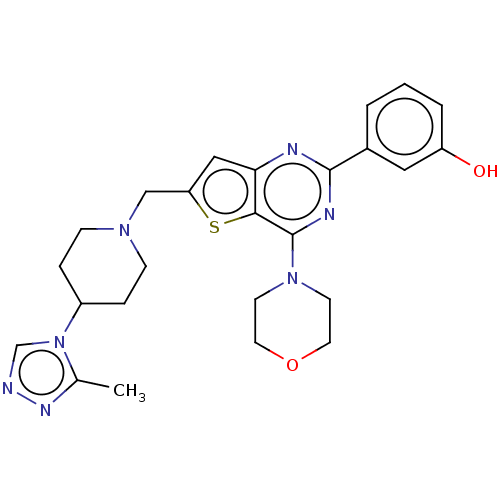
(CHEMBL4068098)Show SMILES Cc1nncn1C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(O)c2)CC1 Show InChI InChI=1S/C25H29N7O2S/c1-17-29-26-16-32(17)19-5-7-30(8-6-19)15-21-14-22-23(35-21)25(31-9-11-34-12-10-31)28-24(27-22)18-3-2-4-20(33)13-18/h2-4,13-14,16,19,33H,5-12,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267166
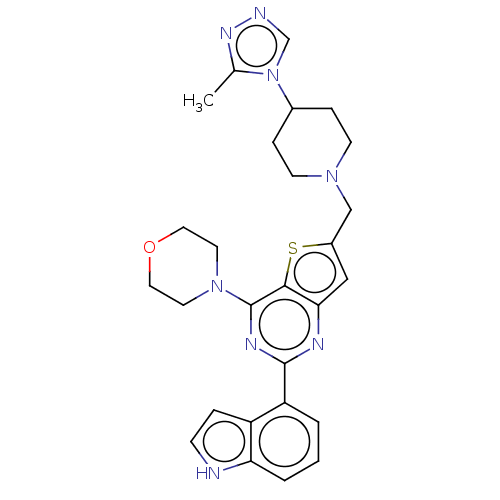
(CHEMBL4105669)Show SMILES Cc1nncn1C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)CC1 Show InChI InChI=1S/C27H30N8OS/c1-18-32-29-17-35(18)19-6-9-33(10-7-19)16-20-15-24-25(37-20)27(34-11-13-36-14-12-34)31-26(30-24)22-3-2-4-23-21(22)5-8-28-23/h2-5,8,15,17,19,28H,6-7,9-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
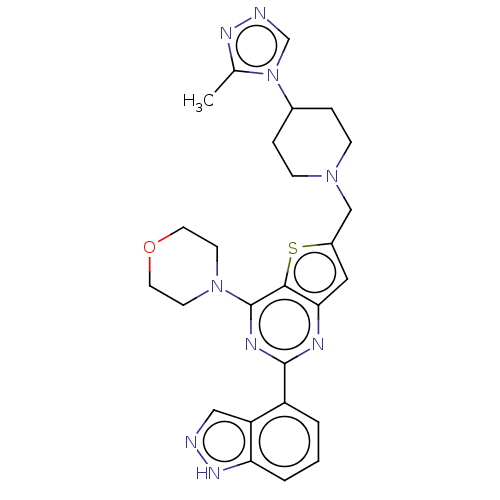
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267134
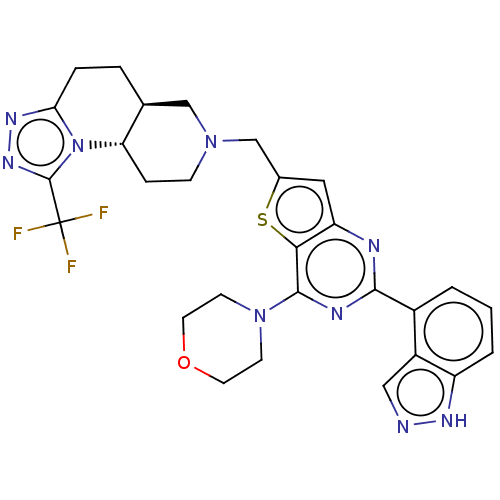
(CHEMBL4092258)Show SMILES [H][C@@]12CCc3nnc(n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc3[nH]ncc13)C2)C(F)(F)F |r| Show InChI InChI=1S/C28H28F3N9OS/c29-28(30,31)27-37-36-23-5-4-16-14-38(7-6-22(16)40(23)27)15-17-12-21-24(42-17)26(39-8-10-41-11-9-39)34-25(33-21)18-2-1-3-20-19(18)13-32-35-20/h1-3,12-13,16,22H,4-11,14-15H2,(H,32,35)/t16-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267165
(CHEMBL4089288)Show SMILES Cc1nncn1C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C26H29N9OS/c1-17-31-28-16-35(17)18-5-7-33(8-6-18)15-19-13-23-24(37-19)26(34-9-11-36-12-10-34)30-25(29-23)20-3-2-4-22-21(20)14-27-32-22/h2-4,13-14,16,18H,5-12,15H2,1H3,(H,27,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267128

(CHEMBL4104716)Show SMILES FC(F)(F)c1nncn1C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)CC1 Show InChI InChI=1S/C27H27F3N8OS/c28-27(29,30)26-35-32-16-38(26)17-5-8-36(9-6-17)15-18-14-22-23(40-18)25(37-10-12-39-13-11-37)34-24(33-22)20-2-1-3-21-19(20)4-7-31-21/h1-4,7,14,16-17,31H,5-6,8-13,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50267159
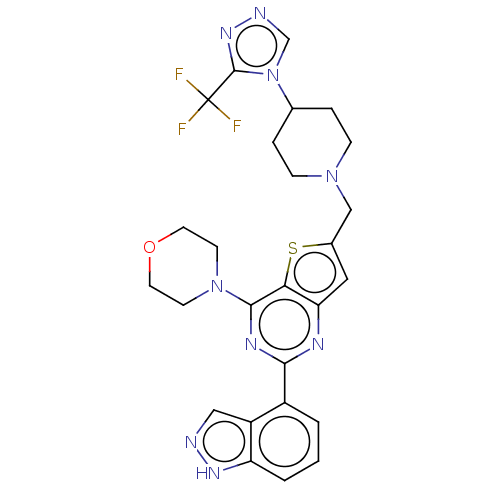
(CHEMBL4086820)Show SMILES FC(F)(F)c1nncn1C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C26H26F3N9OS/c27-26(28,29)25-35-31-15-38(25)16-4-6-36(7-5-16)14-17-12-21-22(40-17)24(37-8-10-39-11-9-37)33-23(32-21)18-2-1-3-20-19(18)13-30-34-20/h1-3,12-13,15-16H,4-11,14H2,(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50086237
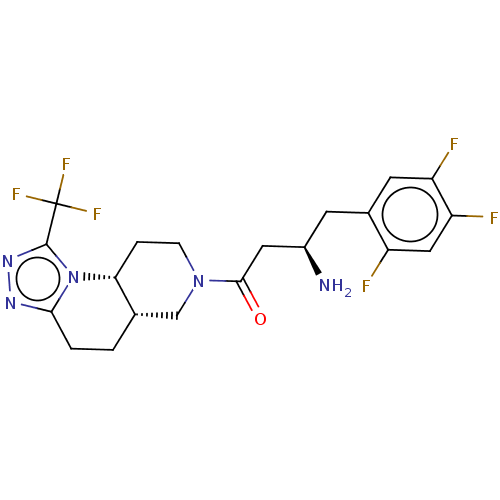
(CHEMBL3425747)Show SMILES [H][C@@]12CCc3nnc(n3[C@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F)C(F)(F)F |r| Show InChI InChI=1S/C20H21F6N5O/c21-13-8-15(23)14(22)6-11(13)5-12(27)7-18(32)30-4-3-16-10(9-30)1-2-17-28-29-19(31(16)17)20(24,25)26/h6,8,10,12,16H,1-5,7,9,27H2/t10-,12+,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50267162
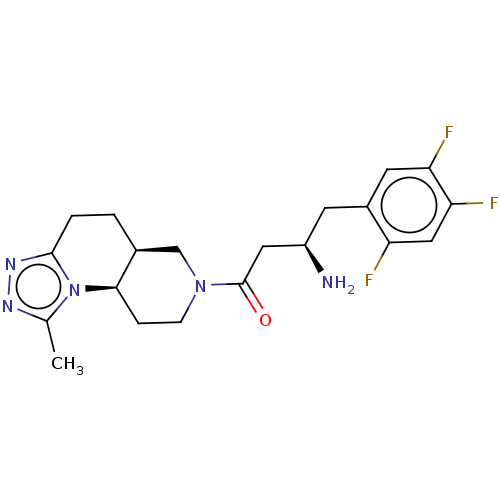
(CHEMBL4063983)Show SMILES Cl.[H][C@@]12CCc3nnc(C)n3[C@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C20H24F3N5O.ClH/c1-11-25-26-19-3-2-12-10-27(5-4-18(12)28(11)19)20(29)8-14(24)6-13-7-16(22)17(23)9-15(13)21;/h7,9,12,14,18H,2-6,8,10,24H2,1H3;1H/t12-,14+,18+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50267162

(CHEMBL4063983)Show SMILES Cl.[H][C@@]12CCc3nnc(C)n3[C@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C20H24F3N5O.ClH/c1-11-25-26-19-3-2-12-10-27(5-4-18(12)28(11)19)20(29)8-14(24)6-13-7-16(22)17(23)9-15(13)21;/h7,9,12,14,18H,2-6,8,10,24H2,1H3;1H/t12-,14+,18+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50086237

(CHEMBL3425747)Show SMILES [H][C@@]12CCc3nnc(n3[C@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F)C(F)(F)F |r| Show InChI InChI=1S/C20H21F6N5O/c21-13-8-15(23)14(22)6-11(13)5-12(27)7-18(32)30-4-3-16-10(9-30)1-2-17-28-29-19(31(16)17)20(24,25)26/h6,8,10,12,16H,1-5,7,9,27H2/t10-,12+,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50267163
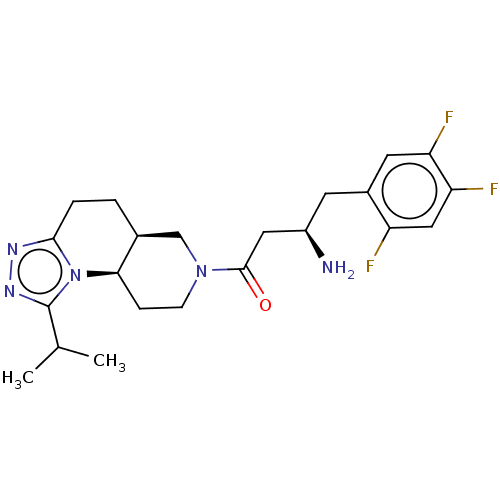
(CHEMBL4085550)Show SMILES Cl.[H][C@@]12CCc3nnc(C(C)C)n3[C@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C22H28F3N5O.ClH/c1-12(2)22-28-27-20-4-3-13-11-29(6-5-19(13)30(20)22)21(31)9-15(26)7-14-8-17(24)18(25)10-16(14)23;/h8,10,12-13,15,19H,3-7,9,11,26H2,1-2H3;1H/t13-,15+,19+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50267120

(CHEMBL4071462)Show SMILES [H][C@@]12CCc3nnc(n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc(O)c1)C2)C(F)(F)F |r| Show InChI InChI=1S/C27H28F3N7O2S/c28-27(29,30)26-34-33-22-5-4-17-14-35(7-6-21(17)37(22)26)15-19-13-20-23(40-19)25(36-8-10-39-11-9-36)32-24(31-20)16-2-1-3-18(38)12-16/h1-3,12-13,17,21,38H,4-11,14-15H2/t17-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50267163

(CHEMBL4085550)Show SMILES Cl.[H][C@@]12CCc3nnc(C(C)C)n3[C@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C22H28F3N5O.ClH/c1-12(2)22-28-27-20-4-3-13-11-29(6-5-19(13)30(20)22)21(31)9-15(26)7-14-8-17(24)18(25)10-16(14)23;/h8,10,12-13,15,19H,3-7,9,11,26H2,1-2H3;1H/t13-,15+,19+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50267179
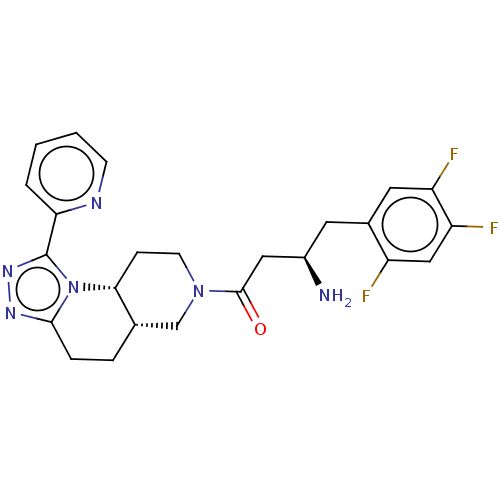
(CHEMBL4062977)Show SMILES Cl.[H][C@@]12CCc3nnc(-c4ccccn4)n3[C@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C24H25F3N6O.ClH/c25-17-12-19(27)18(26)10-15(17)9-16(28)11-23(34)32-8-6-21-14(13-32)4-5-22-30-31-24(33(21)22)20-3-1-2-7-29-20;/h1-3,7,10,12,14,16,21H,4-6,8-9,11,13,28H2;1H/t14-,16+,21+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50267120

(CHEMBL4071462)Show SMILES [H][C@@]12CCc3nnc(n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc(O)c1)C2)C(F)(F)F |r| Show InChI InChI=1S/C27H28F3N7O2S/c28-27(29,30)26-34-33-22-5-4-17-14-35(7-6-21(17)37(22)26)15-19-13-20-23(40-19)25(36-8-10-39-11-9-36)32-24(31-20)16-2-1-3-18(38)12-16/h1-3,12-13,17,21,38H,4-11,14-15H2/t17-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pre... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50267130

(CHEMBL4096987)Show SMILES [H][C@]12CCc3nnc(n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc(O)c1)C2)C(F)(F)F |r| Show InChI InChI=1S/C27H28F3N7O2S/c28-27(29,30)26-34-33-22-5-4-17-14-35(7-6-21(17)37(22)26)15-19-13-20-23(40-19)25(36-8-10-39-11-9-36)32-24(31-20)16-2-1-3-18(38)12-16/h1-3,12-13,17,21,38H,4-11,14-15H2/t17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pre... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50267149
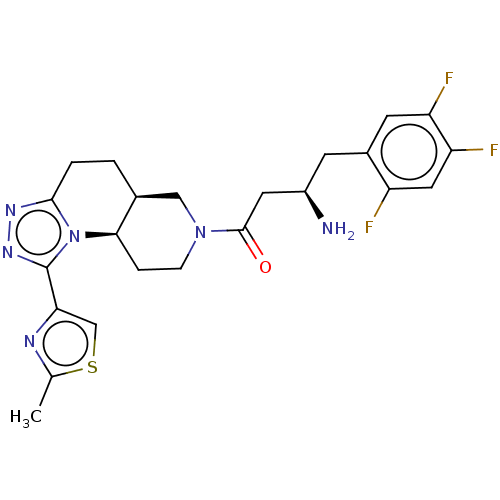
(CHEMBL4071607)Show SMILES Cl.[H][C@@]12CCc3nnc(-c4csc(C)n4)n3[C@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C23H25F3N6OS.ClH/c1-12-28-19(11-34-12)23-30-29-21-3-2-13-10-31(5-4-20(13)32(21)23)22(33)8-15(27)6-14-7-17(25)18(26)9-16(14)24;/h7,9,11,13,15,20H,2-6,8,10,27H2,1H3;1H/t13-,15+,20+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50267148
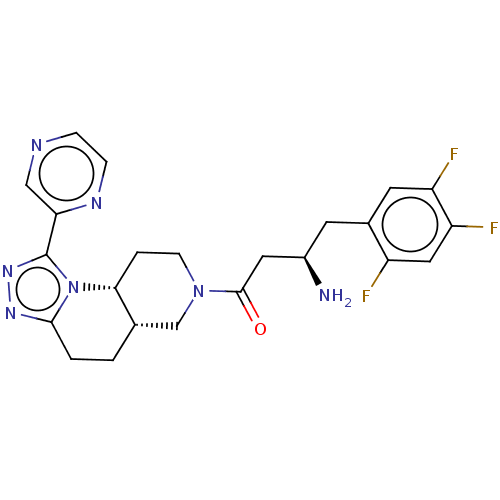
(CHEMBL4090906)Show SMILES Cl.[H][C@@]12CCc3nnc(-c4cnccn4)n3[C@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C23H24F3N7O.ClH/c24-16-10-18(26)17(25)8-14(16)7-15(27)9-22(34)32-6-3-20-13(12-32)1-2-21-30-31-23(33(20)21)19-11-28-4-5-29-19;/h4-5,8,10-11,13,15,20H,1-3,6-7,9,12,27H2;1H/t13-,15+,20+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50267167

(CHEMBL4081413)Show SMILES Oc1cccc(c1)-c1nc(N2CCOCC2)c2sc(CN3CCC(CC3)n3cnnc3C(F)(F)F)cc2n1 Show InChI InChI=1S/C25H26F3N7O2S/c26-25(27,28)24-32-29-15-35(24)17-4-6-33(7-5-17)14-19-13-20-21(38-19)23(34-8-10-37-11-9-34)31-22(30-20)16-2-1-3-18(36)12-16/h1-3,12-13,15,17,36H,4-11,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50267130

(CHEMBL4096987)Show SMILES [H][C@]12CCc3nnc(n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc(O)c1)C2)C(F)(F)F |r| Show InChI InChI=1S/C27H28F3N7O2S/c28-27(29,30)26-34-33-22-5-4-17-14-35(7-6-21(17)37(22)26)15-19-13-20-23(40-19)25(36-8-10-39-11-9-36)32-24(31-20)16-2-1-3-18(38)12-16/h1-3,12-13,17,21,38H,4-11,14-15H2/t17-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50267168
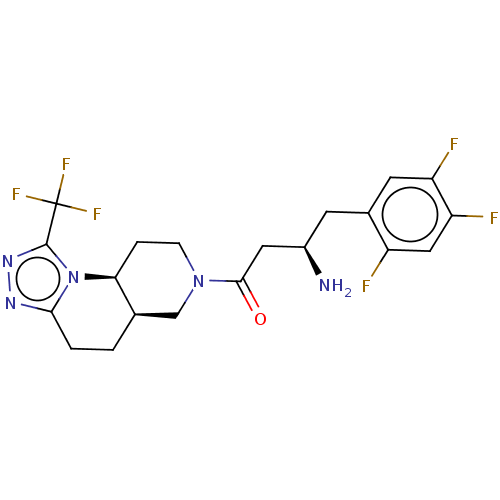
(CHEMBL4066703)Show SMILES [H][C@]12CCc3nnc(n3[C@@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F)C(F)(F)F |r| Show InChI InChI=1S/C20H21F6N5O/c21-13-8-15(23)14(22)6-11(13)5-12(27)7-18(32)30-4-3-16-10(9-30)1-2-17-28-29-19(31(16)17)20(24,25)26/h6,8,10,12,16H,1-5,7,9,27H2/t10-,12-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50267167

(CHEMBL4081413)Show SMILES Oc1cccc(c1)-c1nc(N2CCOCC2)c2sc(CN3CCC(CC3)n3cnnc3C(F)(F)F)cc2n1 Show InChI InChI=1S/C25H26F3N7O2S/c26-25(27,28)24-32-29-15-35(24)17-4-6-33(7-5-17)14-19-13-20-21(38-19)23(34-8-10-37-11-9-34)31-22(30-20)16-2-1-3-18(36)12-16/h1-3,12-13,15,17,36H,4-11,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pre... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50267132

(CHEMBL4068538)Show SMILES [H][C@]12CCc3nnc(n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc3[nH]ccc13)C2)C(F)(F)F |r| Show InChI InChI=1S/C29H29F3N8OS/c30-29(31,32)28-37-36-24-5-4-17-15-38(9-7-23(17)40(24)28)16-18-14-22-25(42-18)27(39-10-12-41-13-11-39)35-26(34-22)20-2-1-3-21-19(20)6-8-33-21/h1-3,6,8,14,17,23,33H,4-5,7,9-13,15-16H2/t17-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pre... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50267164

(CHEMBL4068098)Show SMILES Cc1nncn1C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(O)c2)CC1 Show InChI InChI=1S/C25H29N7O2S/c1-17-29-26-16-32(17)19-5-7-30(8-6-19)15-21-14-22-23(35-21)25(31-9-11-34-12-10-31)28-24(27-22)18-3-2-4-20(33)13-18/h2-4,13-14,16,19,33H,5-12,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50267123

(CHEMBL4094734)Show SMILES [H][C@@]12CCc3nnc(C)n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc(O)c1)C2 |r| Show InChI InChI=1S/C27H31N7O2S/c1-17-30-31-24-6-5-19-15-32(8-7-23(19)34(17)24)16-21-14-22-25(37-21)27(33-9-11-36-12-10-33)29-26(28-22)18-3-2-4-20(35)13-18/h2-4,13-14,19,23,35H,5-12,15-16H2,1H3/t19-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50267177
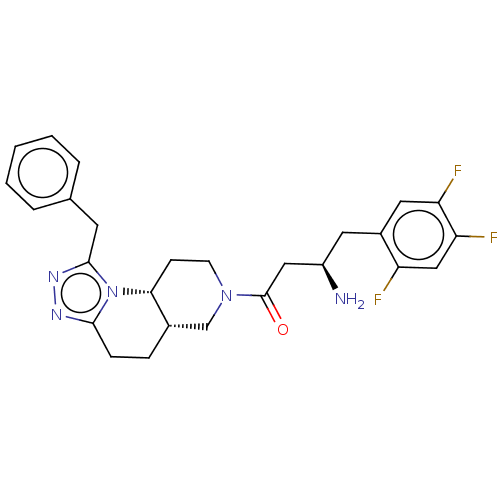
(CHEMBL4077174)Show SMILES Cl.[H][C@@]12CCc3nnc(Cc4ccccc4)n3[C@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C26H28F3N5O.ClH/c27-20-14-22(29)21(28)12-18(20)11-19(30)13-26(35)33-9-8-23-17(15-33)6-7-24-31-32-25(34(23)24)10-16-4-2-1-3-5-16;/h1-5,12,14,17,19,23H,6-11,13,15,30H2;1H/t17-,19+,23+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50267123

(CHEMBL4094734)Show SMILES [H][C@@]12CCc3nnc(C)n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc(O)c1)C2 |r| Show InChI InChI=1S/C27H31N7O2S/c1-17-30-31-24-6-5-19-15-32(8-7-23(19)34(17)24)16-21-14-22-25(37-21)27(33-9-11-36-12-10-33)29-26(28-22)18-3-2-4-20(35)13-18/h2-4,13-14,19,23,35H,5-12,15-16H2,1H3/t19-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pre... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50267178
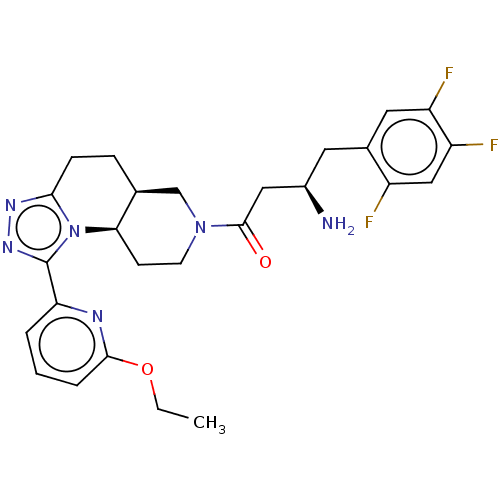
(CHEMBL4093298)Show SMILES Cl.[H][C@@]12CCc3nnc(-c4cccc(OCC)n4)n3[C@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C26H29F3N6O2.ClH/c1-2-37-24-5-3-4-21(31-24)26-33-32-23-7-6-15-14-34(9-8-22(15)35(23)26)25(36)12-17(30)10-16-11-19(28)20(29)13-18(16)27;/h3-5,11,13,15,17,22H,2,6-10,12,14,30H2,1H3;1H/t15-,17+,22+;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50086238
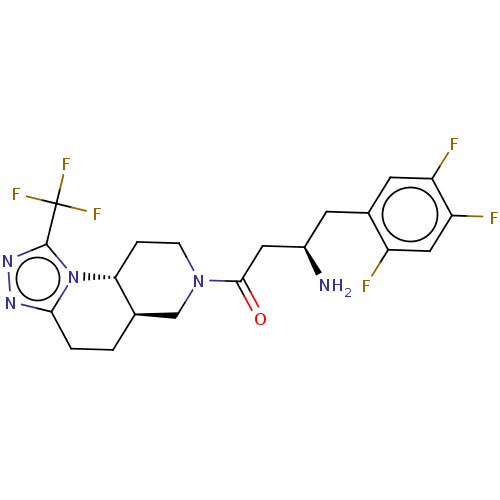
(CHEMBL3425748)Show SMILES [H][C@]12CCc3nnc(n3[C@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F)C(F)(F)F |r| Show InChI InChI=1S/C20H21F6N5O/c21-13-8-15(23)14(22)6-11(13)5-12(27)7-18(32)30-4-3-16-10(9-30)1-2-17-28-29-19(31(16)17)20(24,25)26/h6,8,10,12,16H,1-5,7,9,27H2/t10-,12-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50267158
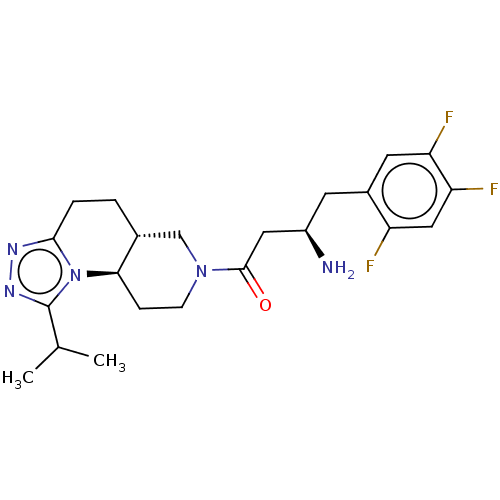
(CHEMBL4098632)Show SMILES Cl.[H][C@]12CCc3nnc(C(C)C)n3[C@]1([H])CCN(C2)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C22H28F3N5O.ClH/c1-12(2)22-28-27-20-4-3-13-11-29(6-5-19(13)30(20)22)21(31)9-15(26)7-14-8-17(24)18(25)10-16(14)23;/h8,10,12-13,15,19H,3-7,9,11,26H2,1-2H3;1H/t13-,15-,19-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 expressed in HEK293 cells using H-Gly-Pro-AMC as substrate measured over 15 mins by fluorescence analysis |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50267134

(CHEMBL4092258)Show SMILES [H][C@@]12CCc3nnc(n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc3[nH]ncc13)C2)C(F)(F)F |r| Show InChI InChI=1S/C28H28F3N9OS/c29-28(30,31)27-37-36-23-5-4-16-14-38(7-6-22(16)40(23)27)15-17-12-21-24(42-17)26(39-8-10-41-11-9-39)34-25(33-21)18-2-1-3-20-19(18)13-32-35-20/h1-3,12-13,16,22H,4-11,14-15H2,(H,32,35)/t16-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50267164

(CHEMBL4068098)Show SMILES Cc1nncn1C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(O)c2)CC1 Show InChI InChI=1S/C25H29N7O2S/c1-17-29-26-16-32(17)19-5-7-30(8-6-19)15-21-14-22-23(35-21)25(31-9-11-34-12-10-31)28-24(27-22)18-3-2-4-20(33)13-18/h2-4,13-14,16,19,33H,5-12,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pre... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50267121

(CHEMBL4070417)Show SMILES [H][C@@]12CCc3nnc(C)n3[C@@]1([H])CCN(Cc1cc3nc(nc(N4CCOCC4)c3s1)-c1cccc3[nH]ncc13)C2 |r| Show InChI InChI=1S/C28H31N9OS/c1-17-32-34-25-6-5-18-15-35(8-7-24(18)37(17)25)16-19-13-23-26(39-19)28(36-9-11-38-12-10-36)31-27(30-23)20-3-2-4-22-21(20)14-29-33-22/h2-4,13-14,18,24H,5-12,15-16H2,1H3,(H,29,33)/t18-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50267167

(CHEMBL4081413)Show SMILES Oc1cccc(c1)-c1nc(N2CCOCC2)c2sc(CN3CCC(CC3)n3cnnc3C(F)(F)F)cc2n1 Show InChI InChI=1S/C25H26F3N7O2S/c26-25(27,28)24-32-29-15-35(24)17-4-6-33(7-5-17)14-19-13-20-21(38-19)23(34-8-10-37-11-9-34)31-22(30-20)16-2-1-3-18(36)12-16/h1-3,12-13,15,17,36H,4-11,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
University Park Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate preincubated for 15 mins followed by substrate addition measured after 60 mins in pr... |
J Med Chem 60: 1534-1554 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01801
BindingDB Entry DOI: 10.7270/Q2251MPR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data