 Found 101 hits Enz. Inhib. hit(s) with all data for entry = 50001274
Found 101 hits Enz. Inhib. hit(s) with all data for entry = 50001274 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50274638
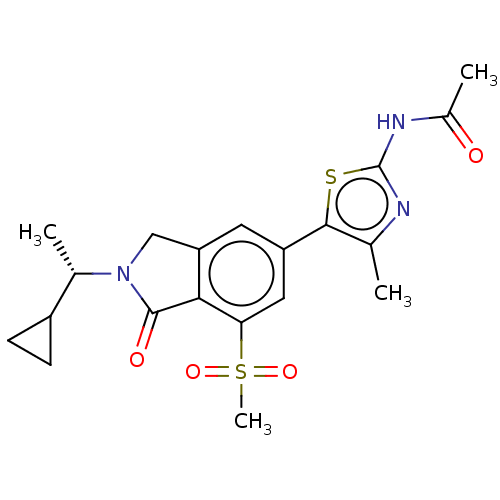
(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human P2Y1 receptor |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274660
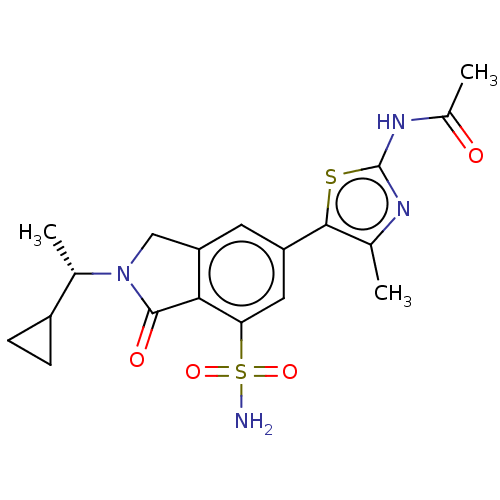
(CHEMBL4128537 | US10858355, Example 12)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(N)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C19H22N4O4S2/c1-9-17(28-19(21-9)22-11(3)24)13-6-14-8-23(10(2)12-4-5-12)18(25)16(14)15(7-13)29(20,26)27/h6-7,10,12H,4-5,8H2,1-3H3,(H2,20,26,27)(H,21,22,24)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274640
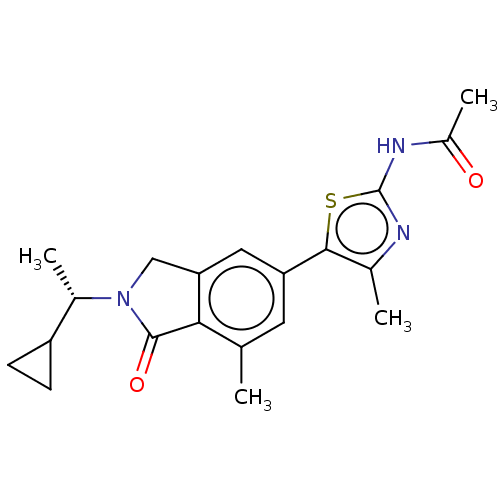
(CHEMBL4126445)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O2S/c1-10-7-15(18-11(2)21-20(26-18)22-13(4)24)8-16-9-23(19(25)17(10)16)12(3)14-5-6-14/h7-8,12,14H,5-6,9H2,1-4H3,(H,21,22,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274648
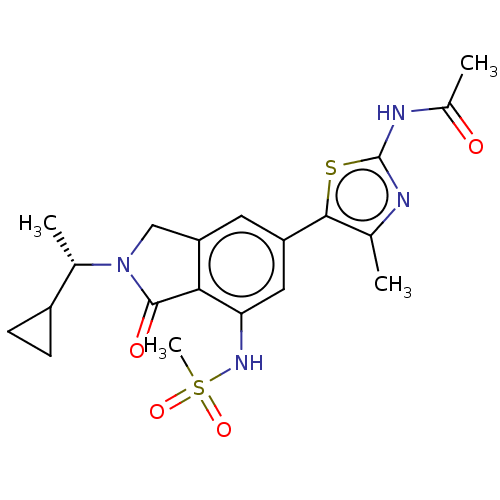
(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274649
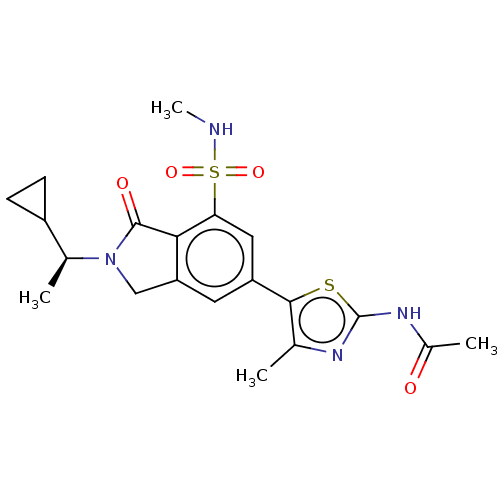
(CHEMBL4129600 | US10858355, Example 30)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(22-10)23-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)30(27,28)21-4/h7-8,11,13,21H,5-6,9H2,1-4H3,(H,22,23,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274675
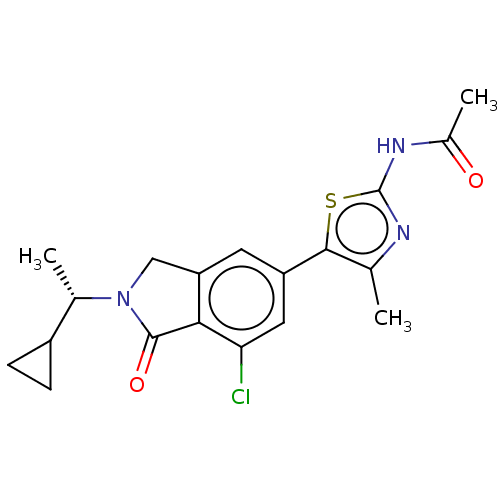
(CHEMBL4126601)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C19H20ClN3O2S/c1-9-17(26-19(21-9)22-11(3)24)13-6-14-8-23(10(2)12-4-5-12)18(25)16(14)15(20)7-13/h6-7,10,12H,4-5,8H2,1-3H3,(H,21,22,24)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274641
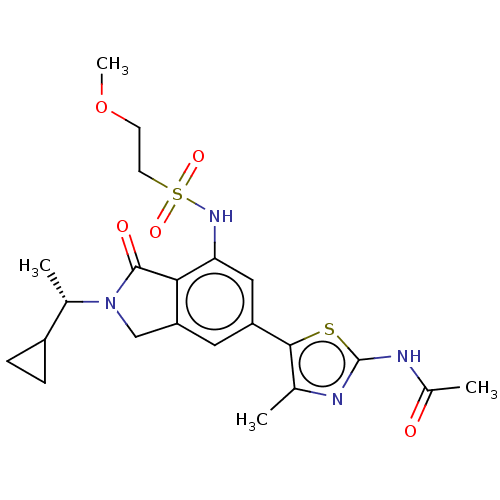
(CHEMBL4126010 | US10858355, Example 27)Show SMILES COCCS(=O)(=O)Nc1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H28N4O5S2/c1-12-20(32-22(23-12)24-14(3)27)16-9-17-11-26(13(2)15-5-6-15)21(28)19(17)18(10-16)25-33(29,30)8-7-31-4/h9-10,13,15,25H,5-8,11H2,1-4H3,(H,23,24,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274639

(CHEMBL4128230 | US10858355, Example 18)Show SMILES COCCNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H28N4O5S2/c1-12-20(32-22(24-12)25-14(3)27)16-9-17-11-26(13(2)15-5-6-15)21(28)19(17)18(10-16)33(29,30)23-7-8-31-4/h9-10,13,15,23H,5-8,11H2,1-4H3,(H,24,25,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274655
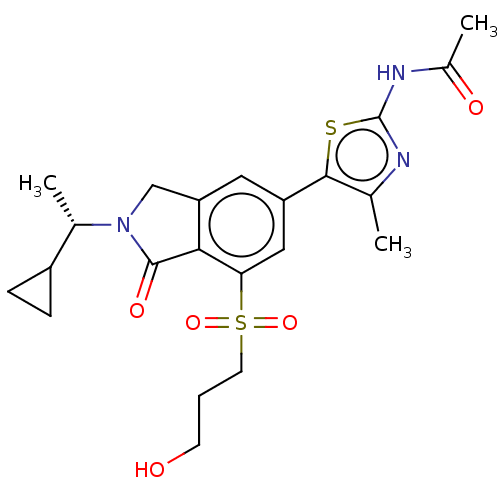
(CHEMBL4129457)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)CCCO)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H27N3O5S2/c1-12-20(31-22(23-12)24-14(3)27)16-9-17-11-25(13(2)15-5-6-15)21(28)19(17)18(10-16)32(29,30)8-4-7-26/h9-10,13,15,26H,4-8,11H2,1-3H3,(H,23,24,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274642
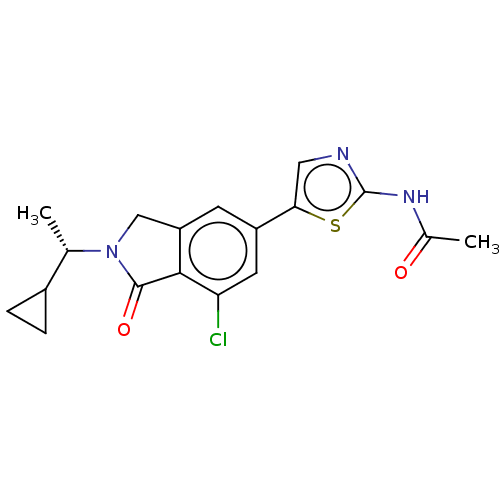
(CHEMBL4129877)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1cnc(NC(C)=O)s1 |r| Show InChI InChI=1S/C18H18ClN3O2S/c1-9(11-3-4-11)22-8-13-5-12(6-14(19)16(13)17(22)24)15-7-20-18(25-15)21-10(2)23/h5-7,9,11H,3-4,8H2,1-2H3,(H,20,21,23)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274657
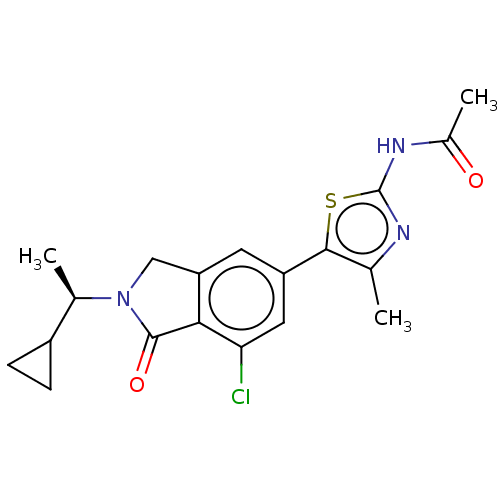
(CHEMBL4127833)Show SMILES C[C@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C19H20ClN3O2S/c1-9-17(26-19(21-9)22-11(3)24)13-6-14-8-23(10(2)12-4-5-12)18(25)16(14)15(20)7-13/h6-7,10,12H,4-5,8H2,1-3H3,(H,21,22,24)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274649

(CHEMBL4129600 | US10858355, Example 30)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(22-10)23-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)30(27,28)21-4/h7-8,11,13,21H,5-6,9H2,1-4H3,(H,22,23,25)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274656
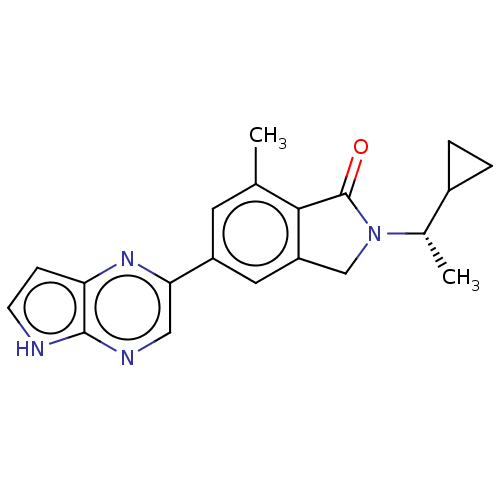
(CHEMBL4128652)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1cnc2[nH]ccc2n1 |r| Show InChI InChI=1S/C20H20N4O/c1-11-7-14(17-9-22-19-16(23-17)5-6-21-19)8-15-10-24(20(25)18(11)15)12(2)13-3-4-13/h5-9,12-13H,3-4,10H2,1-2H3,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274641

(CHEMBL4126010 | US10858355, Example 27)Show SMILES COCCS(=O)(=O)Nc1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H28N4O5S2/c1-12-20(32-22(23-12)24-14(3)27)16-9-17-11-26(13(2)15-5-6-15)21(28)19(17)18(10-16)25-33(29,30)8-7-31-4/h9-10,13,15,25H,5-8,11H2,1-4H3,(H,23,24,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274640

(CHEMBL4126445)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O2S/c1-10-7-15(18-11(2)21-20(26-18)22-13(4)24)8-16-9-23(19(25)17(10)16)12(3)14-5-6-14/h7-8,12,14H,5-6,9H2,1-4H3,(H,21,22,24)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274675

(CHEMBL4126601)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C19H20ClN3O2S/c1-9-17(26-19(21-9)22-11(3)24)13-6-14-8-23(10(2)12-4-5-12)18(25)16(14)15(20)7-13/h6-7,10,12H,4-5,8H2,1-3H3,(H,21,22,24)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274650
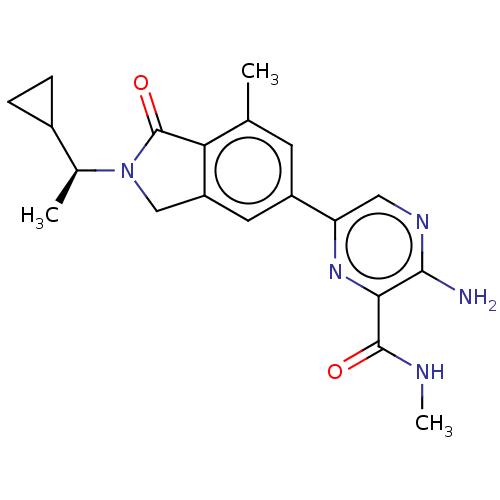
(CHEMBL4130021)Show SMILES CNC(=O)c1nc(cnc1N)-c1cc2CN([C@@H](C)C3CC3)C(=O)c2c(C)c1 |r| Show InChI InChI=1S/C20H23N5O2/c1-10-6-13(15-8-23-18(21)17(24-15)19(26)22-3)7-14-9-25(20(27)16(10)14)11(2)12-4-5-12/h6-8,11-12H,4-5,9H2,1-3H3,(H2,21,23)(H,22,26)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274638

(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274674
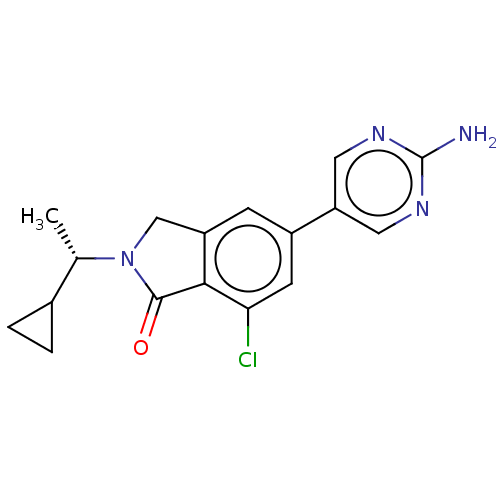
(CHEMBL4127414)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C17H17ClN4O/c1-9(10-2-3-10)22-8-12-4-11(5-14(18)15(12)16(22)23)13-6-20-17(19)21-7-13/h4-7,9-10H,2-3,8H2,1H3,(H2,19,20,21)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 17A
(Homo sapiens (Human)) | BDBM50274640

(CHEMBL4126445)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O2S/c1-10-7-15(18-11(2)21-20(26-18)22-13(4)24)8-16-9-23(19(25)17(10)16)12(3)14-5-6-14/h7-8,12,14H,5-6,9H2,1-4H3,(H,21,22,24)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length GST-tagged DRAK1 expressed in baculovirus expression system |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274639

(CHEMBL4128230 | US10858355, Example 18)Show SMILES COCCNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H28N4O5S2/c1-12-20(32-22(24-12)25-14(3)27)16-9-17-11-26(13(2)15-5-6-15)21(28)19(17)18(10-16)33(29,30)23-7-8-31-4/h9-10,13,15,23H,5-8,11H2,1-4H3,(H,24,25,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274660

(CHEMBL4128537 | US10858355, Example 12)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(N)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C19H22N4O4S2/c1-9-17(28-19(21-9)22-11(3)24)13-6-14-8-23(10(2)12-4-5-12)18(25)16(14)15(7-13)29(20,26)27/h6-7,10,12H,4-5,8H2,1-3H3,(H2,20,26,27)(H,21,22,24)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274658
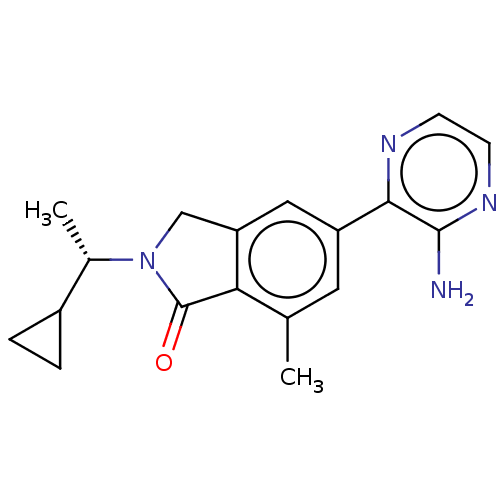
(CHEMBL4128809)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1nccnc1N |r| Show InChI InChI=1S/C18H20N4O/c1-10-7-13(16-17(19)21-6-5-20-16)8-14-9-22(18(23)15(10)14)11(2)12-3-4-12/h5-8,11-12H,3-4,9H2,1-2H3,(H2,19,21)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50274638

(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3KC2beta (unknown origin) |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274642

(CHEMBL4129877)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1cnc(NC(C)=O)s1 |r| Show InChI InChI=1S/C18H18ClN3O2S/c1-9(11-3-4-11)22-8-13-5-12(6-14(19)16(13)17(22)24)15-7-20-18(25-15)21-10(2)23/h5-7,9,11H,3-4,8H2,1-2H3,(H,20,21,23)/t9-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274657

(CHEMBL4127833)Show SMILES C[C@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C19H20ClN3O2S/c1-9-17(26-19(21-9)22-11(3)24)13-6-14-8-23(10(2)12-4-5-12)18(25)16(14)15(20)7-13/h6-7,10,12H,4-5,8H2,1-3H3,(H,21,22,24)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274647
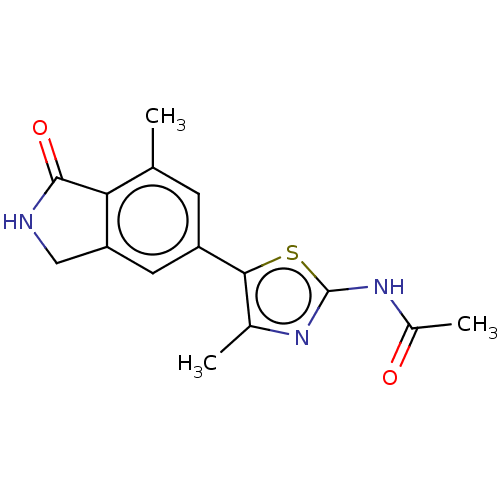
(CHEMBL4127150)Show InChI InChI=1S/C15H15N3O2S/c1-7-4-10(5-11-6-16-14(20)12(7)11)13-8(2)17-15(21-13)18-9(3)19/h4-5H,6H2,1-3H3,(H,16,20)(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50274647

(CHEMBL4127150)Show InChI InChI=1S/C15H15N3O2S/c1-7-4-10(5-11-6-16-14(20)12(7)11)13-8(2)17-15(21-13)18-9(3)19/h4-5H,6H2,1-3H3,(H,16,20)(H,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110alpha/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK4
(Homo sapiens (Human)) | BDBM50274640

(CHEMBL4126445)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O2S/c1-10-7-15(18-11(2)21-20(26-18)22-13(4)24)8-16-9-23(19(25)17(10)16)12(3)14-5-6-14/h7-8,12,14H,5-6,9H2,1-4H3,(H,21,22,24)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length GST-tagged CLK4 expressed in baculovirus expression system |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274649

(CHEMBL4129600 | US10858355, Example 30)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(22-10)23-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)30(27,28)21-4/h7-8,11,13,21H,5-6,9H2,1-4H3,(H,22,23,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274676
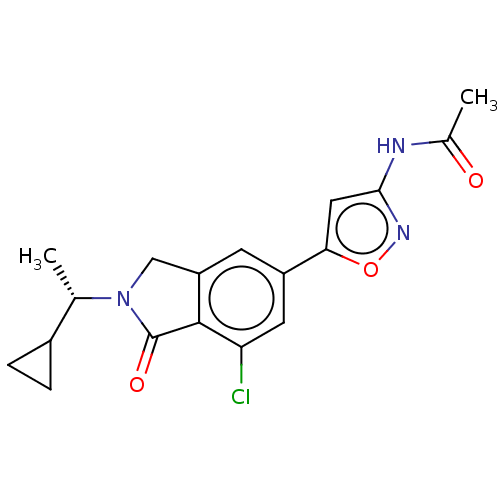
(CHEMBL4125955)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1cc(NC(C)=O)no1 |r| Show InChI InChI=1S/C18H18ClN3O3/c1-9(11-3-4-11)22-8-13-5-12(6-14(19)17(13)18(22)24)15-7-16(21-25-15)20-10(2)23/h5-7,9,11H,3-4,8H2,1-2H3,(H,20,21,23)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274655

(CHEMBL4129457)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)CCCO)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H27N3O5S2/c1-12-20(31-22(23-12)24-14(3)27)16-9-17-11-25(13(2)15-5-6-15)21(28)19(17)18(10-16)32(29,30)8-4-7-26/h9-10,13,15,26H,4-8,11H2,1-3H3,(H,23,24,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274640

(CHEMBL4126445)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O2S/c1-10-7-15(18-11(2)21-20(26-18)22-13(4)24)8-16-9-23(19(25)17(10)16)12(3)14-5-6-14/h7-8,12,14H,5-6,9H2,1-4H3,(H,21,22,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274647

(CHEMBL4127150)Show InChI InChI=1S/C15H15N3O2S/c1-7-4-10(5-11-6-16-14(20)12(7)11)13-8(2)17-15(21-13)18-9(3)19/h4-5H,6H2,1-3H3,(H,16,20)(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274655

(CHEMBL4129457)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)CCCO)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H27N3O5S2/c1-12-20(31-22(23-12)24-14(3)27)16-9-17-11-25(13(2)15-5-6-15)21(28)19(17)18(10-16)32(29,30)8-4-7-26/h9-10,13,15,26H,4-8,11H2,1-3H3,(H,23,24,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274641

(CHEMBL4126010 | US10858355, Example 27)Show SMILES COCCS(=O)(=O)Nc1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H28N4O5S2/c1-12-20(32-22(23-12)24-14(3)27)16-9-17-11-26(13(2)15-5-6-15)21(28)19(17)18(10-16)25-33(29,30)8-7-31-4/h9-10,13,15,25H,5-8,11H2,1-4H3,(H,23,24,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274660

(CHEMBL4128537 | US10858355, Example 12)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(N)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C19H22N4O4S2/c1-9-17(28-19(21-9)22-11(3)24)13-6-14-8-23(10(2)12-4-5-12)18(25)16(14)15(7-13)29(20,26)27/h6-7,10,12H,4-5,8H2,1-3H3,(H2,20,26,27)(H,21,22,24)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274638

(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274675

(CHEMBL4126601)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C19H20ClN3O2S/c1-9-17(26-19(21-9)22-11(3)24)13-6-14-8-23(10(2)12-4-5-12)18(25)16(14)15(20)7-13/h6-7,10,12H,4-5,8H2,1-3H3,(H,21,22,24)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274639

(CHEMBL4128230 | US10858355, Example 18)Show SMILES COCCNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H28N4O5S2/c1-12-20(32-22(24-12)25-14(3)27)16-9-17-11-26(13(2)15-5-6-15)21(28)19(17)18(10-16)33(29,30)23-7-8-31-4/h9-10,13,15,23H,5-8,11H2,1-4H3,(H,24,25,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274650

(CHEMBL4130021)Show SMILES CNC(=O)c1nc(cnc1N)-c1cc2CN([C@@H](C)C3CC3)C(=O)c2c(C)c1 |r| Show InChI InChI=1S/C20H23N5O2/c1-10-6-13(15-8-23-18(21)17(24-15)19(26)22-3)7-14-9-25(20(27)16(10)14)11(2)12-4-5-12/h6-8,11-12H,4-5,9H2,1-3H3,(H2,21,23)(H,22,26)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274649

(CHEMBL4129600 | US10858355, Example 30)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(22-10)23-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)30(27,28)21-4/h7-8,11,13,21H,5-6,9H2,1-4H3,(H,22,23,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in human JeKo1B cells assessed as reduction in AKT phosphorylation at ser473 residue preincubated for 60 mins followed by ant... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274656

(CHEMBL4128652)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1cnc2[nH]ccc2n1 |r| Show InChI InChI=1S/C20H20N4O/c1-11-7-14(17-9-22-19-16(23-17)5-6-21-19)8-15-10-24(20(25)18(11)15)12(2)13-3-4-13/h5-9,12-13H,3-4,10H2,1-2H3,(H,21,22)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274657

(CHEMBL4127833)Show SMILES C[C@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C19H20ClN3O2S/c1-9-17(26-19(21-9)22-11(3)24)13-6-14-8-23(10(2)12-4-5-12)18(25)16(14)15(20)7-13/h6-7,10,12H,4-5,8H2,1-3H3,(H,21,22,24)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3K p110delta/p85alpha using DiC8-PIP2 as substrate preincubated for 10 mins followed by substrate addit... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50274674

(CHEMBL4127414)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C17H17ClN4O/c1-9(10-2-3-10)22-8-12-4-11(5-14(18)15(12)16(22)23)13-6-20-17(19)21-7-13/h4-7,9-10H,2-3,8H2,1H3,(H2,19,20,21)/t9-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in human JeKo1B cells assessed as reduction in AKT phosphorylation at ser473 residue preincubated for 60 mins followed by ant... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274659
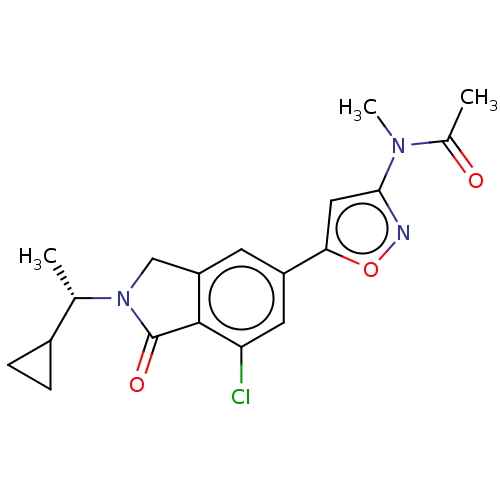
(CHEMBL4129270)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1cc(no1)N(C)C(C)=O |r| Show InChI InChI=1S/C19H20ClN3O3/c1-10(12-4-5-12)23-9-14-6-13(7-15(20)18(14)19(23)25)16-8-17(21-26-16)22(3)11(2)24/h6-8,10,12H,4-5,9H2,1-3H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50274638

(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in human JeKo1B cells assessed as reduction in AKT phosphorylation at ser473 residue preincubated for 60 mins followed by ant... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data