

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407474 (CHEMBL3137917) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407473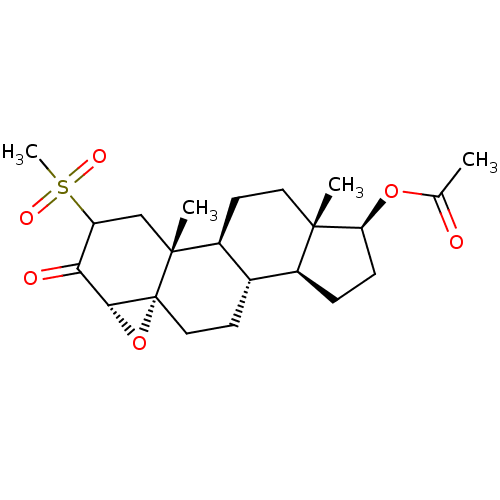 (CHEMBL3137919) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407488 (CHEMBL3137916) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407477 (CHEMBL109492) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407490 (CHEMBL3137910) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50032828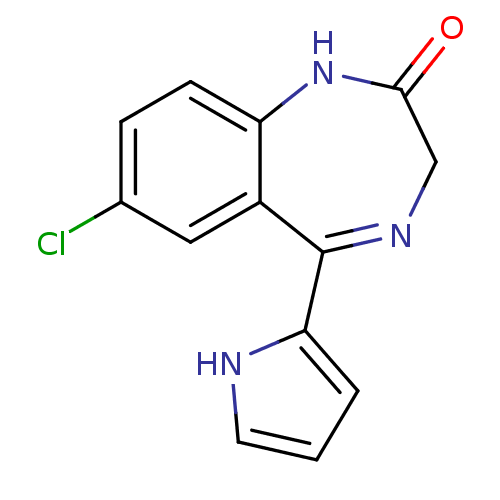 (7-Chloro-5-(1H-pyrrol-2-yl)-1,3-dihydro-benzo[e][1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407479 (CHEMBL323490) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407471 (CHEMBL320506) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407487 (CHEMBL2373330) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407480 (CHEMBL3137920) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407483 (CHEMBL108427) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407472 (CHEMBL3137913) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407475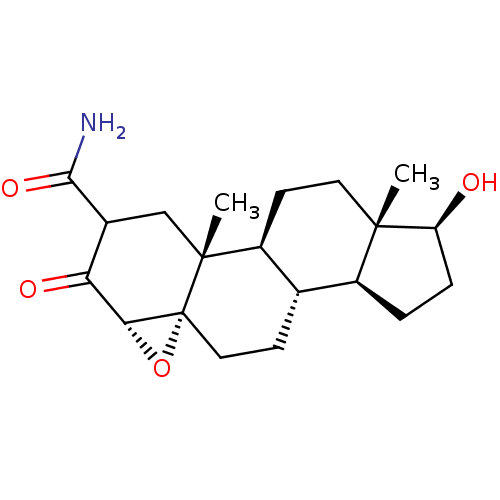 (CHEMBL3137911) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407485 (CHEMBL3137914) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407482 (CHEMBL107878) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407486 (CHEMBL108485) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407478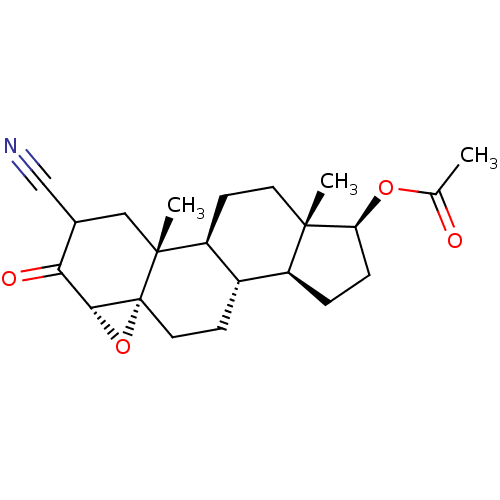 (CHEMBL3138234) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407481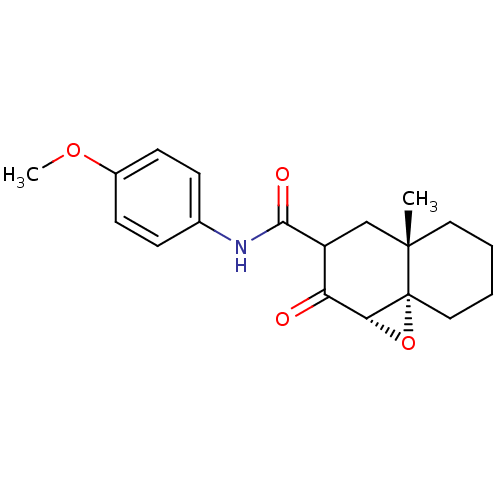 (CHEMBL111446) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407489 (CHEMBL111424) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407469 (CHEMBL323217) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407476 (CHEMBL325765) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407468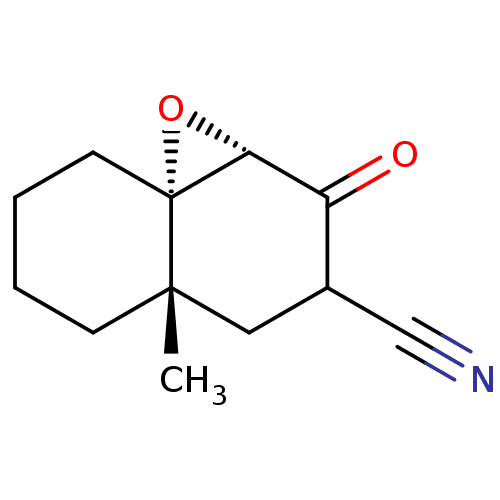 (CHEMBL110573) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407484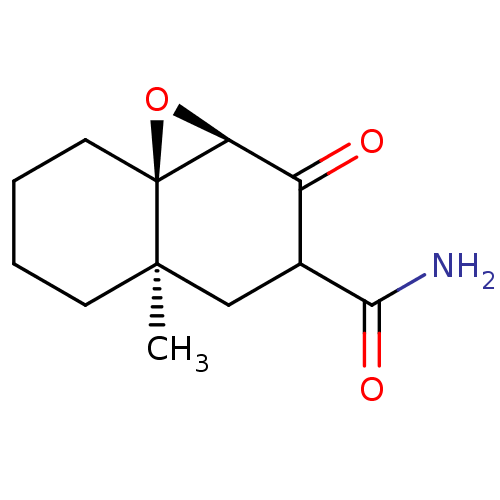 (CHEMBL320265) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Tat (Human immunodeficiency virus type 1 (isolate PCV12...) | BDBM50407470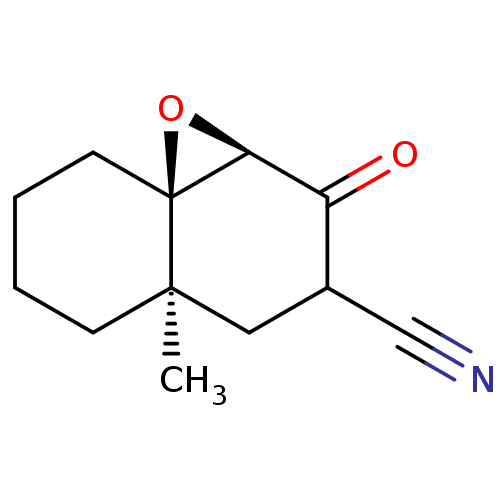 (CHEMBL326009) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaecuticals Research Division Curated by ChEMBL | Assay Description The compound was tested for inhibition of HIV-1 replication in SW480 cells using HIV tat assay | J Med Chem 38: 3197-206 (1995) BindingDB Entry DOI: 10.7270/Q27D2WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||