 Found 11 hits Enz. Inhib. hit(s) with all data for entry = 50037217
Found 11 hits Enz. Inhib. hit(s) with all data for entry = 50037217 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50135920
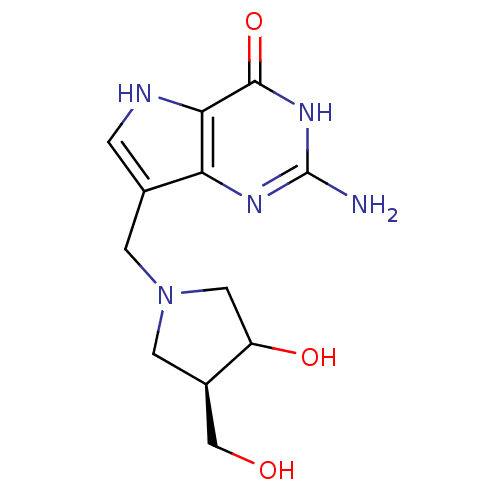
(2-Amino-7-(3-hydroxy-4-hydroxymethyl-pyrrolidin-1-...)Show SMILES Nc1nc2c(CN3CC(O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22109
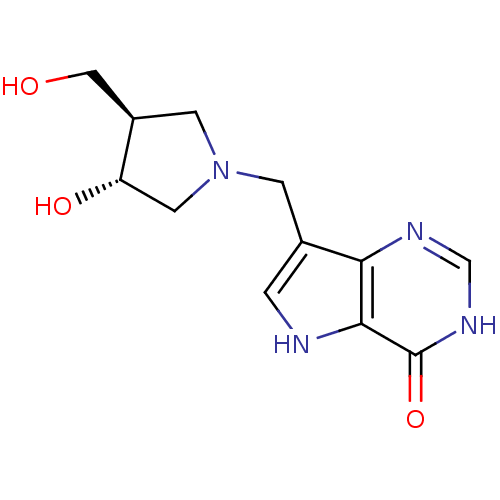
(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293086
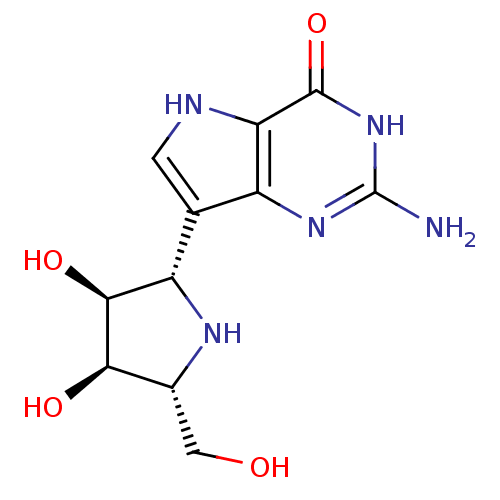
((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O4/c12-11-15-5-3(1-13-7(5)10(20)16-11)6-9(19)8(18)4(2-17)14-6/h1,4,6,8-9,13-14,17-19H,2H2,(H3,12,15,16,20)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50135920

(2-Amino-7-(3-hydroxy-4-hydroxymethyl-pyrrolidin-1-...)Show SMILES Nc1nc2c(CN3CC(O)[C@@H](CO)C3)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(1-14-10(9)11(20)16-12)2-17-3-7(5-18)8(19)4-17/h1,7-8,14,18-19H,2-5H2,(H3,13,15,16,20)/t7-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity towards Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50422435
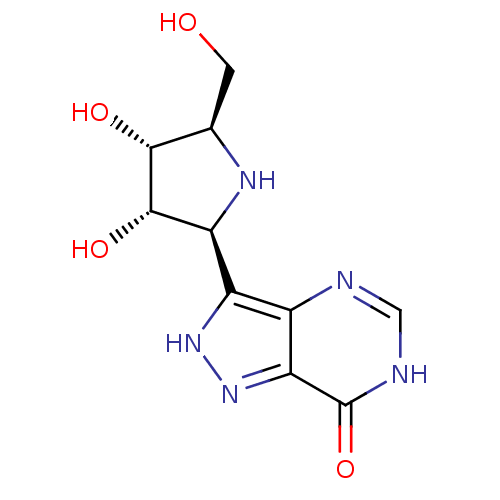
(CHEMBL2311112)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1[nH]nc2c1nc[nH]c2=O Show InChI InChI=1S/C10H13N5O4/c16-1-3-8(17)9(18)6(13-3)5-4-7(15-14-5)10(19)12-2-11-4/h2-3,6,8-9,13,16-18H,1H2,(H,14,15)(H,11,12,19)/t3-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Dissociation constant against Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50293086

((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H]1N[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H15N5O4/c12-11-15-5-3(1-13-7(5)10(20)16-11)6-9(19)8(18)4(2-17)14-6/h1,4,6,8-9,13-14,17-19H,2H2,(H3,12,15,16,20)/t4-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity towards Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM22109

(7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...)Show SMILES OC[C@H]1CN(Cc2c[nH]c3c2nc[nH]c3=O)C[C@@H]1O |r| Show InChI InChI=1S/C12H16N4O3/c17-5-8-3-16(4-9(8)18)2-7-1-13-11-10(7)14-6-15-12(11)19/h1,6,8-9,13,17-18H,2-5H2,(H,14,15,19)/t8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity towards Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50422435

(CHEMBL2311112)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1[nH]nc2c1nc[nH]c2=O Show InChI InChI=1S/C10H13N5O4/c16-1-3-8(17)9(18)6(13-3)5-4-7(15-14-5)10(19)12-2-11-4/h2-3,6,8-9,13,16-18H,1H2,(H,14,15)(H,11,12,19)/t3-,6+,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity towards Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50135919

(3-(3-Hydroxy-4-hydroxymethyl-pyrrolidin-1-ylmethyl...)Show InChI InChI=1S/C11H15N5O3/c17-4-6-1-16(3-8(6)18)2-7-9-10(15-14-7)11(19)13-5-12-9/h5-6,8,17-18H,1-4H2,(H,14,15)(H,12,13,19)/t6-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity towards Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited
Curated by ChEMBL
| Assay Description
Binding affinity towards Human Purine Nucleoside Phosphorylase was reported |
J Med Chem 46: 5271-6 (2003)
Article DOI: 10.1021/jm030305z
BindingDB Entry DOI: 10.7270/Q23N2449 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data