

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 103 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Agonist activity at human LXRbeta-LBD assessed as recruitment of co-activator peptide after 2 hrs by TR-FRET assay | Bioorg Med Chem Lett 23: 4185-90 (2013) Article DOI: 10.1016/j.bmcl.2013.05.030 BindingDB Entry DOI: 10.7270/Q2NK3GF4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50241503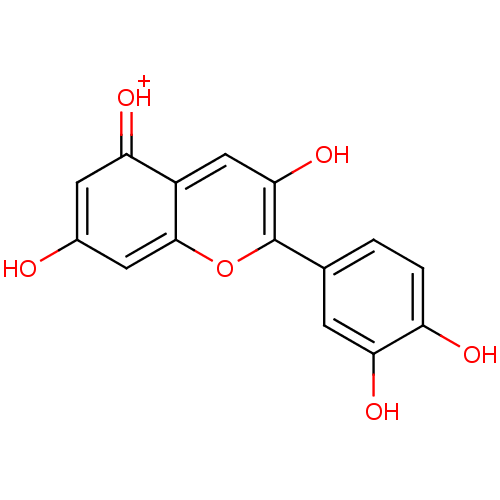 (2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-1-Benzopy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Agonist activity at human LXRbeta-LBD assessed as recruitment of co-activator peptide after 2 hrs by TR-FRET assay | Bioorg Med Chem Lett 23: 4185-90 (2013) Article DOI: 10.1016/j.bmcl.2013.05.030 BindingDB Entry DOI: 10.7270/Q2NK3GF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20177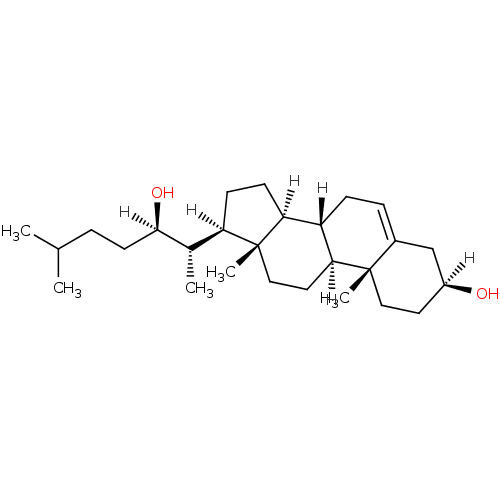 ((1S,2R,5S,10S,11S,14R,15S)-14-[(2S,3R)-3-hydroxy-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Agonist activity at human LXRalpha-LBD assessed as recruitment of co-activator peptide after 2 hrs by TR-FRET assay | Bioorg Med Chem Lett 23: 4185-90 (2013) Article DOI: 10.1016/j.bmcl.2013.05.030 BindingDB Entry DOI: 10.7270/Q2NK3GF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 91 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Agonist activity at human LXRalpha-LBD assessed as recruitment of co-activator peptide after 2 hrs by TR-FRET assay | Bioorg Med Chem Lett 23: 4185-90 (2013) Article DOI: 10.1016/j.bmcl.2013.05.030 BindingDB Entry DOI: 10.7270/Q2NK3GF4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50241503 (2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-1-Benzopy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Binding affinity to human LXRalpha-LBD by surface plasmon resonance | Bioorg Med Chem Lett 23: 4185-90 (2013) Article DOI: 10.1016/j.bmcl.2013.05.030 BindingDB Entry DOI: 10.7270/Q2NK3GF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Binding affinity to human LXRbeta-LBD by surface plasmon resonance | Bioorg Med Chem Lett 23: 4185-90 (2013) Article DOI: 10.1016/j.bmcl.2013.05.030 BindingDB Entry DOI: 10.7270/Q2NK3GF4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50241503 (2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-1-Benzopy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 7.32E+4 | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Binding affinity to human LXRbeta-LBD by surface plasmon resonance | Bioorg Med Chem Lett 23: 4185-90 (2013) Article DOI: 10.1016/j.bmcl.2013.05.030 BindingDB Entry DOI: 10.7270/Q2NK3GF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Binding affinity to human LXRalpha-LBD by surface plasmon resonance | Bioorg Med Chem Lett 23: 4185-90 (2013) Article DOI: 10.1016/j.bmcl.2013.05.030 BindingDB Entry DOI: 10.7270/Q2NK3GF4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50241503 (2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-1-Benzopy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Agonist activity at human LXRalpha-LBD assessed as recruitment of co-activator peptide after 2 hrs by TR-FRET assay | Bioorg Med Chem Lett 23: 4185-90 (2013) Article DOI: 10.1016/j.bmcl.2013.05.030 BindingDB Entry DOI: 10.7270/Q2NK3GF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||