

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438864 (CHEMBL2420507) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438848 (CHEMBL2420480) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438854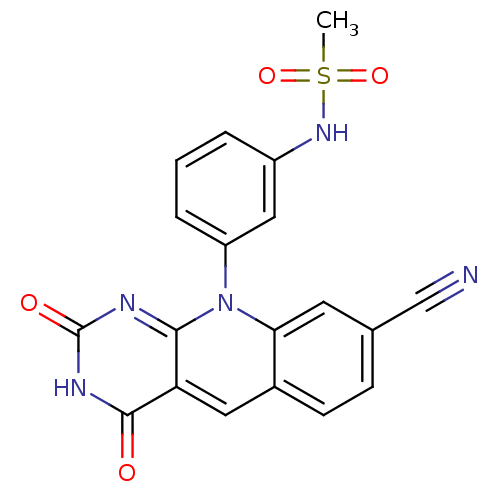 (CHEMBL2420474) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM81348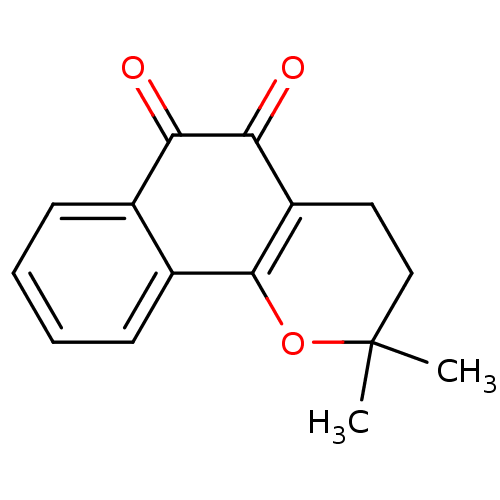 (β-Lapachone (A3) | Beta lapachone | R115 (Rea...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 4-nitrophenyl phenylphosphonate as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438863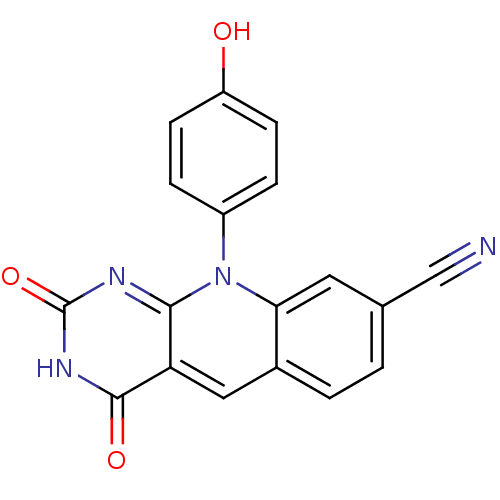 (10-(4-Hydroxyphenyl)-2,4-dioxo-pyrimido[4,5-b]quin...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50438913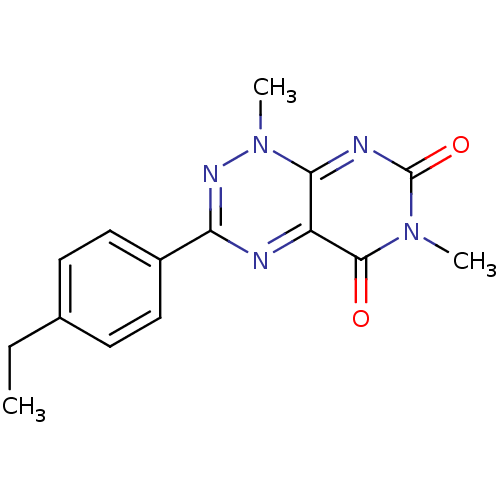 (CHEMBL2420615) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM34905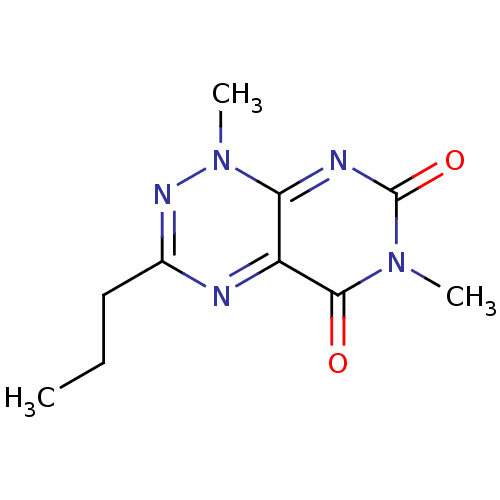 (1,6-dimethyl-3-propyl-pyrimido[5,4-e][1,2,4]triazi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438859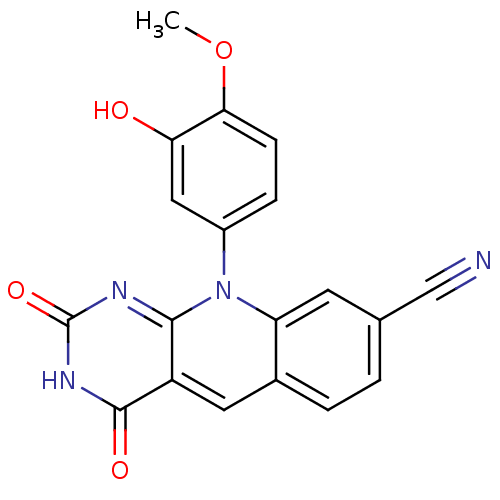 (CHEMBL2420469) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438868 (CHEMBL2420503) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50396070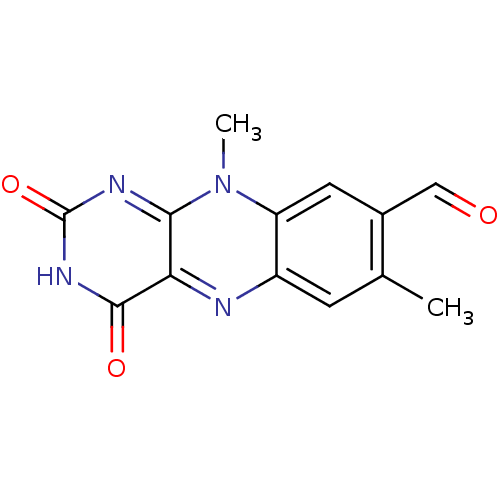 (CHEMBL1397270) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 4-nitrophenyl phenylphosphonate as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438850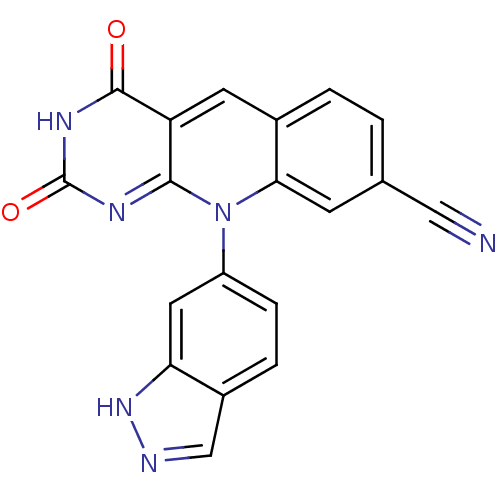 (CHEMBL2420478) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438865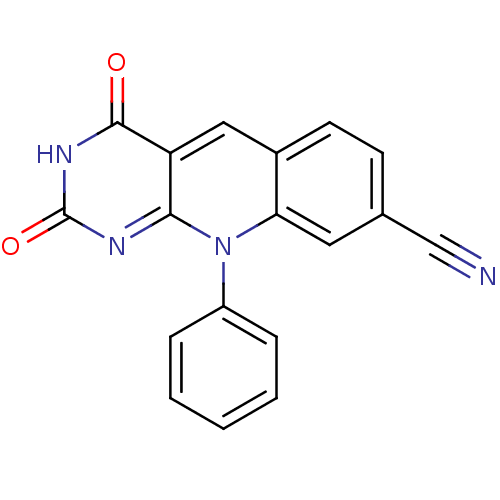 (2,4-Dioxo-10-phenyl-pyrimido[4,5-b]quinoline-8-car...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438849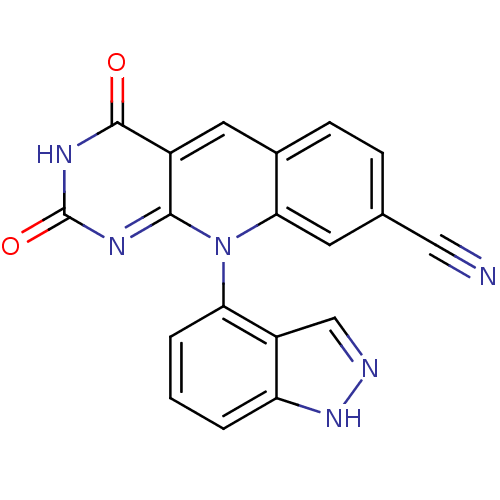 (CHEMBL2420479) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438870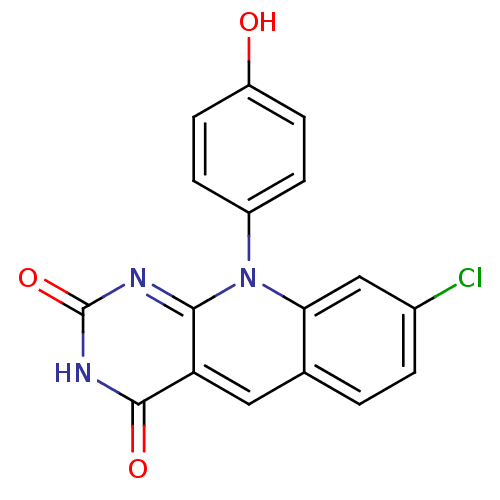 (8-Chloro-10-(4-hydroxyphenyl)pyrimido[4,5-b]quinol...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438857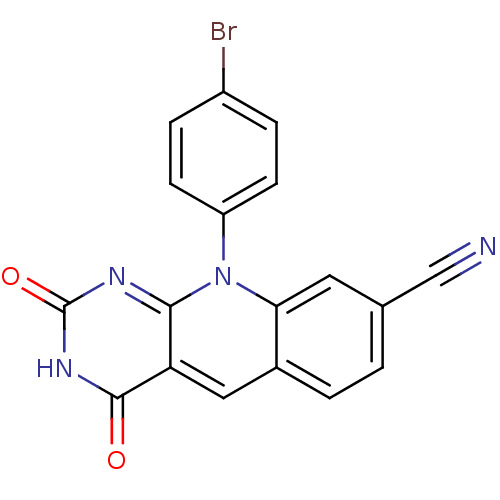 (CHEMBL2420471) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438866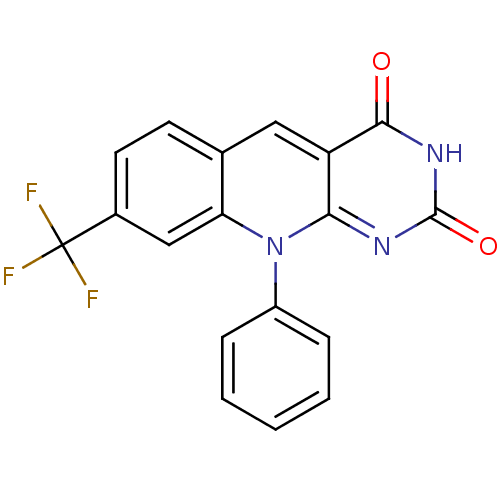 (CHEMBL2420505) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438867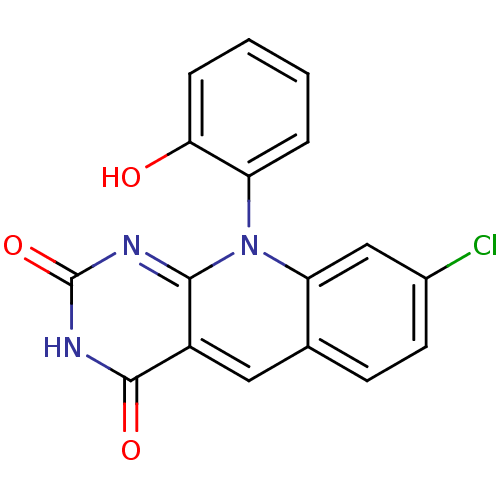 (CHEMBL2420504) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438882 (10-Phenylpyrimido[4,5-b]quinoline-2,4(3H,10H)-dion...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438881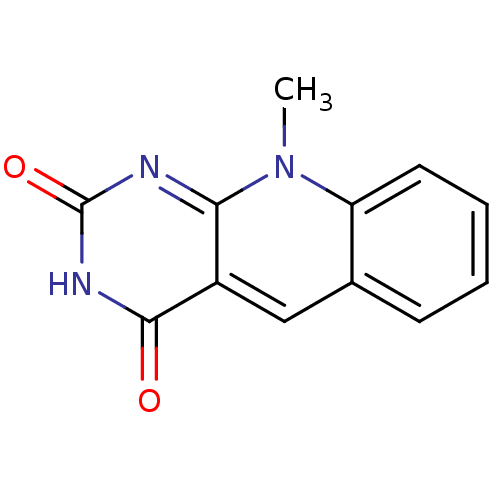 (CHEMBL228772) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438880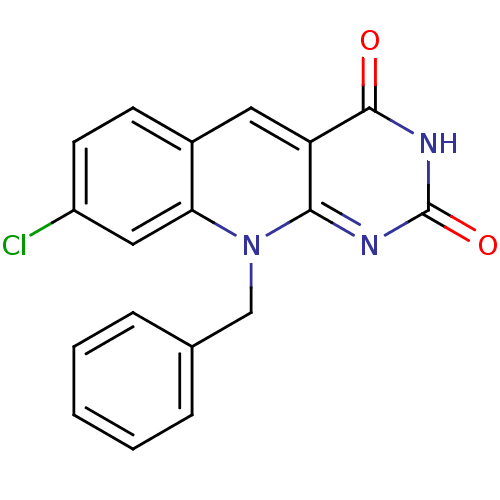 (CHEMBL2420492) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438879 (CHEMBL2420493) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438874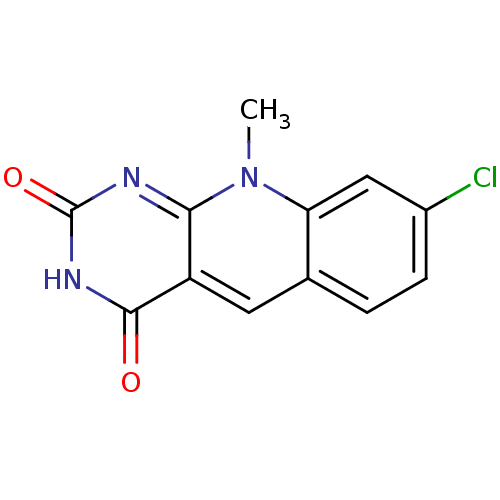 (CHEMBL2420497) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438872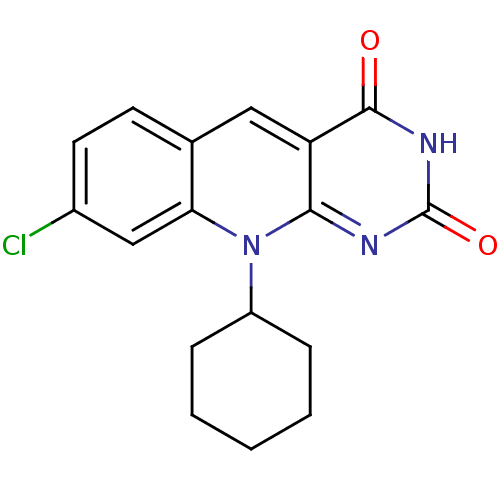 (CHEMBL2420499) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438869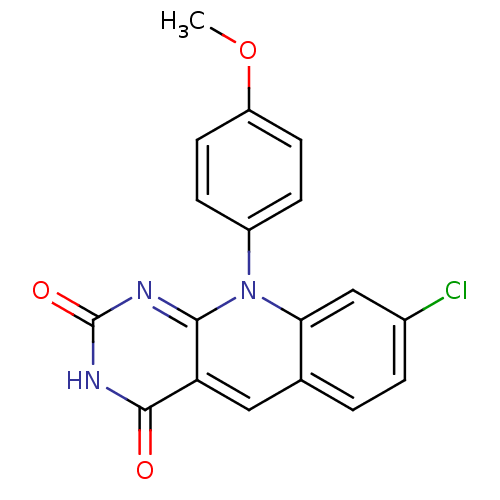 (CHEMBL2420502) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438871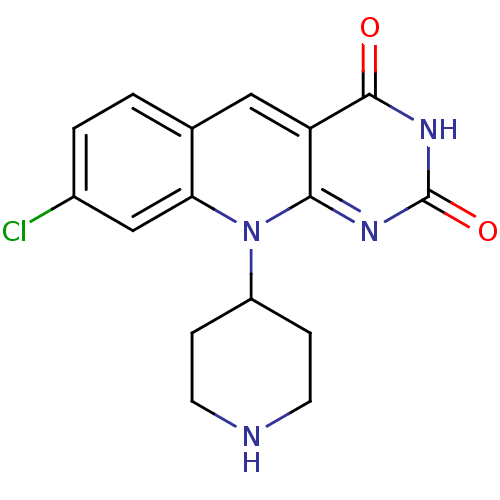 (CHEMBL2420500) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 5'-Y-TCCGTTGAAGCCTGCTTT-3' as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50208827 (2,3-bis(2-hydroxyethylthio)naphthalene-1,4-dione |...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of TDP2 (unknown origin) using 4-nitrophenyl phenylphosphonate as substrate after 60 mins | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438863 (10-(4-Hydroxyphenyl)-2,4-dioxo-pyrimido[4,5-b]quin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438864 (CHEMBL2420507) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438865 (2,4-Dioxo-10-phenyl-pyrimido[4,5-b]quinoline-8-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438866 (CHEMBL2420505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438867 (CHEMBL2420504) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438868 (CHEMBL2420503) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438869 (CHEMBL2420502) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438870 (8-Chloro-10-(4-hydroxyphenyl)pyrimido[4,5-b]quinol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438871 (CHEMBL2420500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438872 (CHEMBL2420499) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438873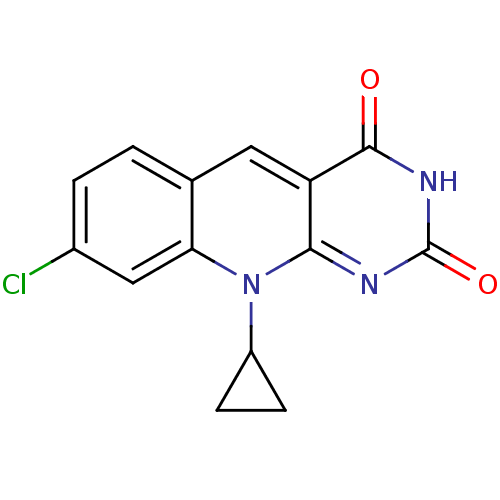 (CHEMBL2420498) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438874 (CHEMBL2420497) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438875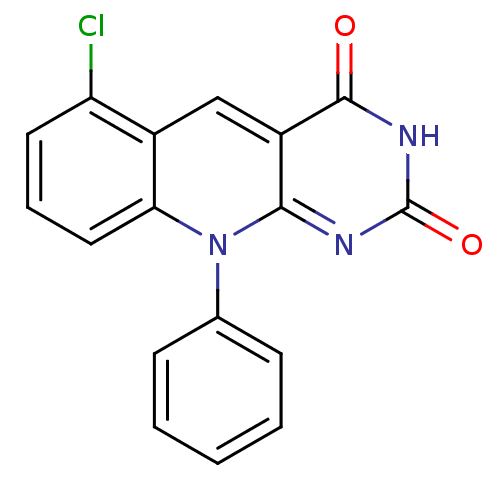 (CHEMBL224548) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438876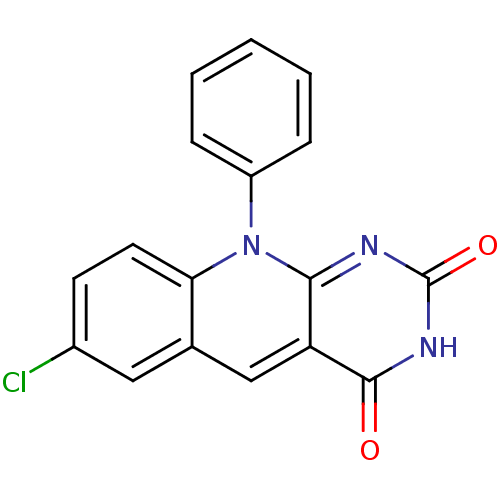 (CHEMBL2420496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438877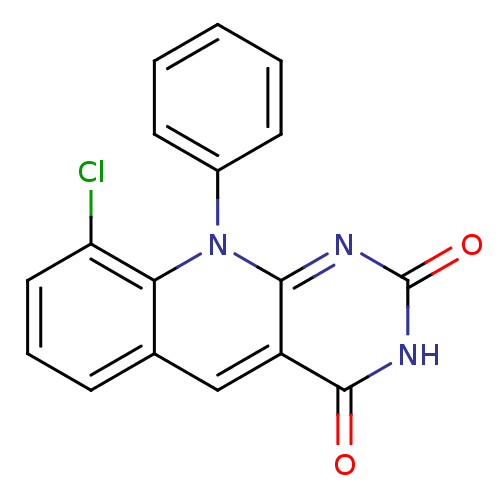 (CHEMBL2420495) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438878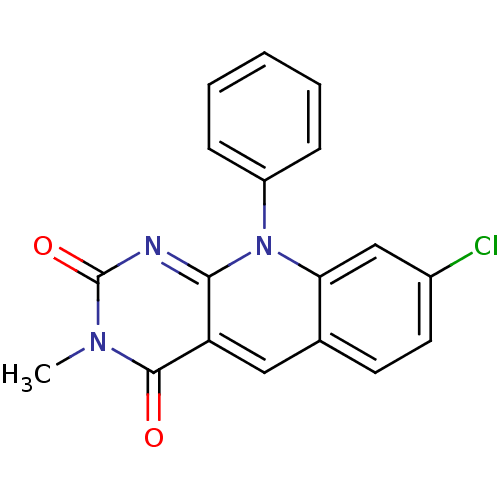 (CHEMBL2420494) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438879 (CHEMBL2420493) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438880 (CHEMBL2420492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438881 (CHEMBL228772) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438882 (10-Phenylpyrimido[4,5-b]quinoline-2,4(3H,10H)-dion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438883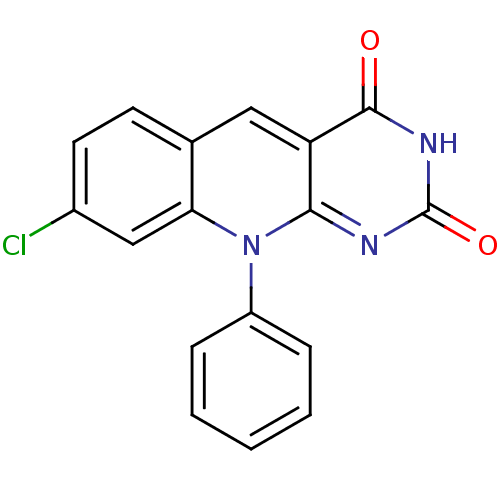 (CHEMBL223823) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438884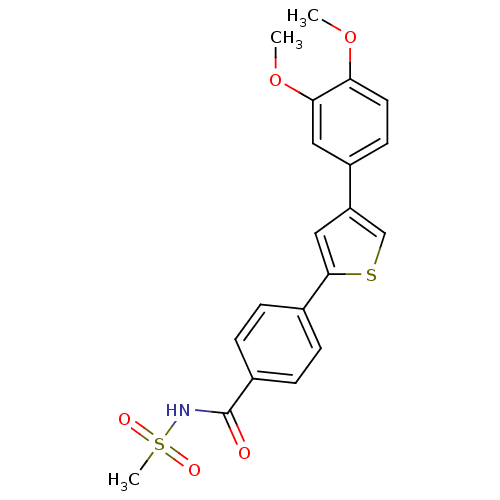 (CHEMBL2420491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438885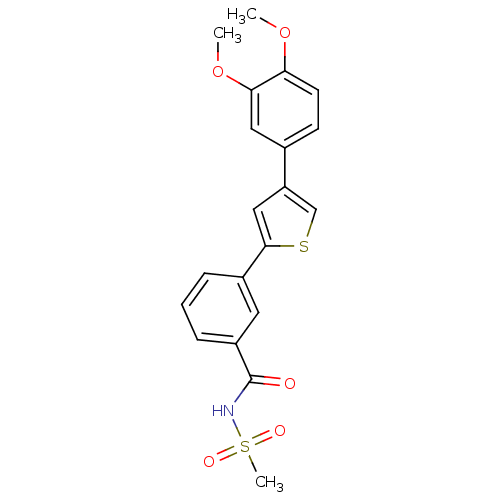 (CHEMBL2420490) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-(apurinic or apyrimidinic site) endonuclease (Homo sapiens (Human)) | BDBM50438886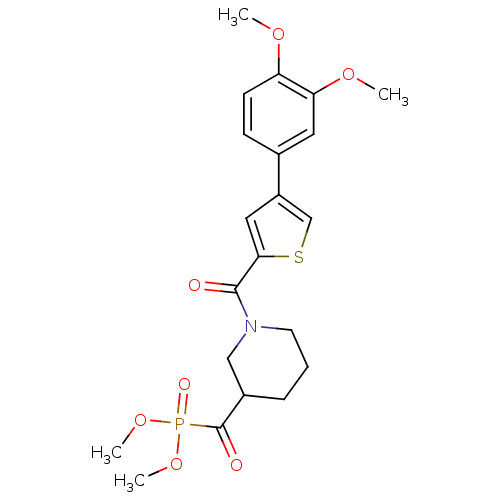 (CHEMBL2420489) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of APE-1 (unknown origin) using double-stranded AP-site containing DNA as substrate after 30 mins by fluorescence assay | J Med Chem 56: 6352-70 (2013) Article DOI: 10.1021/jm400568p BindingDB Entry DOI: 10.7270/Q2N017ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 189 total ) | Next | Last >> |