

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human PR expressed in CHO-K1 cells assessed as reduction in progesterone-induced response incubated for 20 hrs by luciferase r... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368944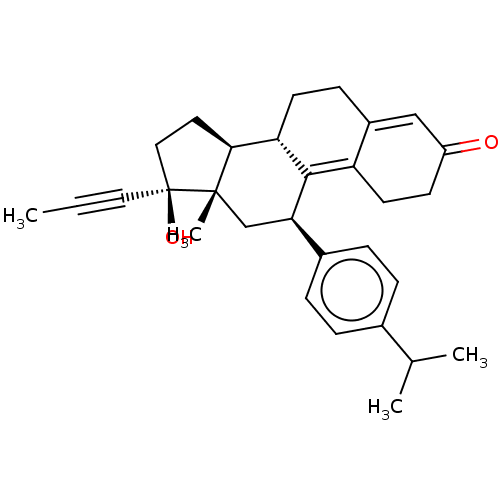 (CHEMBL4172624) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368971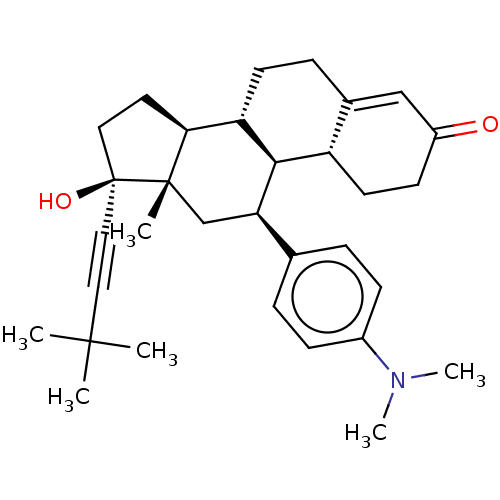 (CHEMBL4159987) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368933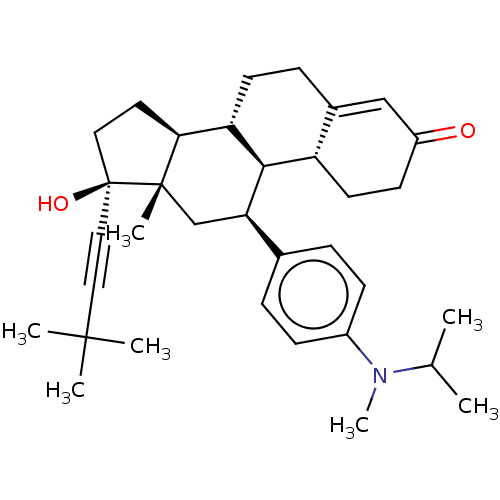 (CHEMBL4161754) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368960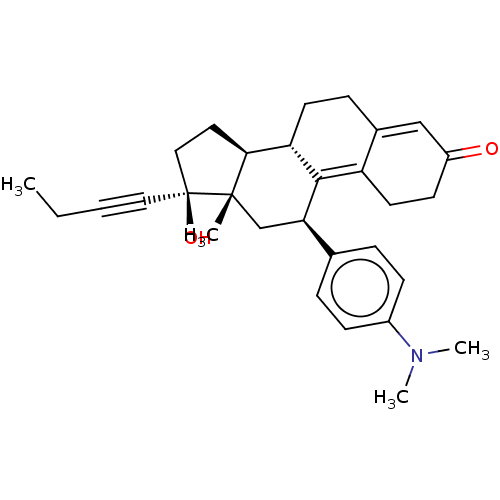 (CHEMBL4159692) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368924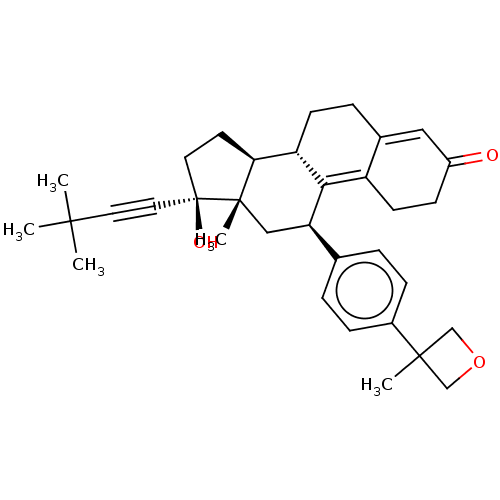 (CHEMBL4167905 | US11124537, TABLE 3.47.1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368949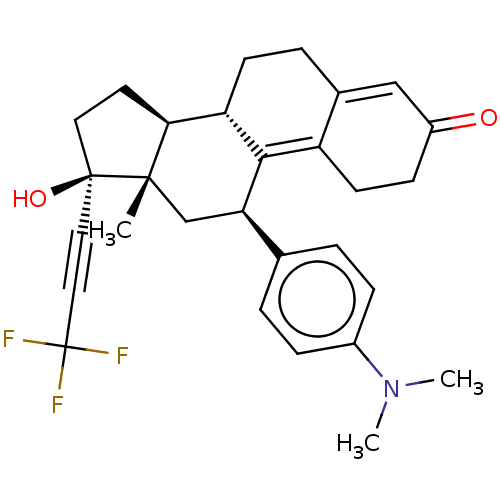 (CHEMBL4164157) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Sus scrofa) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in mini pig PBMC assessed as reduction in dexamethasone-induced GILZ gene expression incubated for 24 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368966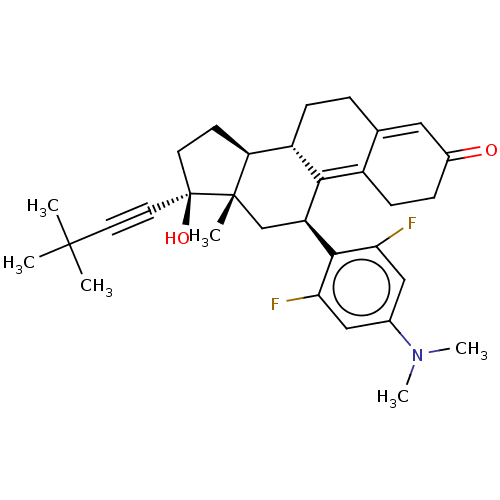 (CHEMBL4164900 | US11124537, TABLE 3.20.2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in human OVCAR5 assessed as reduction in dexamethasone-induced FKBP5 gene expression incubated for 24 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368957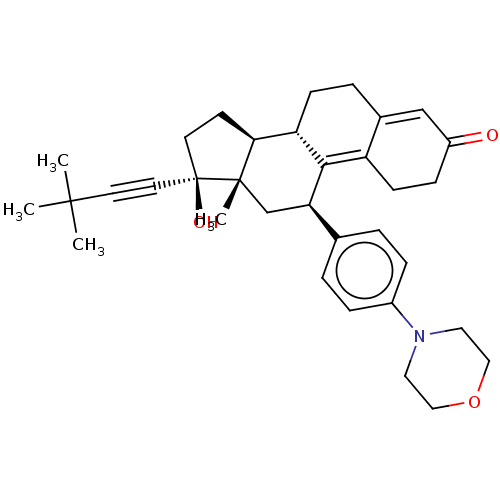 (CHEMBL4171957) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368936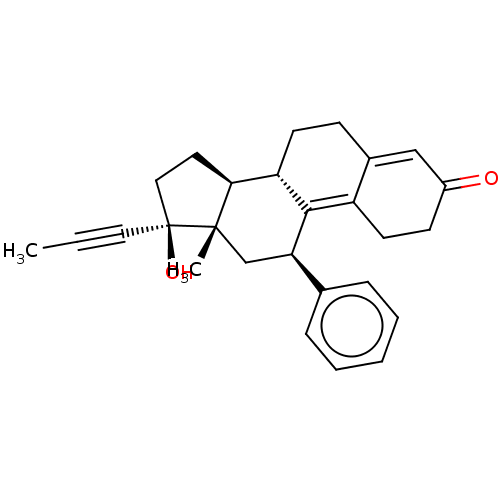 (CHEMBL4159430) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368945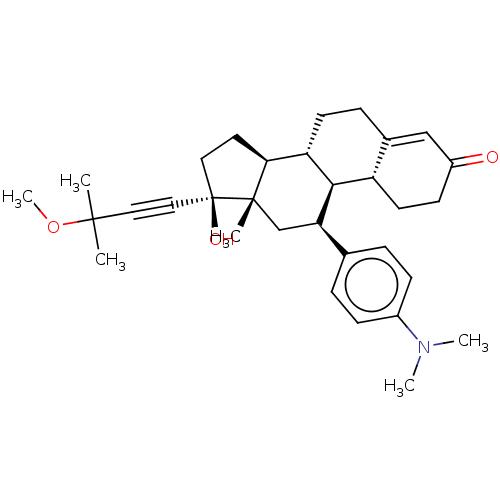 (CHEMBL4169685) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in human PBMC assessed as reduction in dexamethasone-induced FKBP5 gene expression incubated for 6 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368977 (CHEMBL4163069 | US11124537, TABLE 3.31.1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368925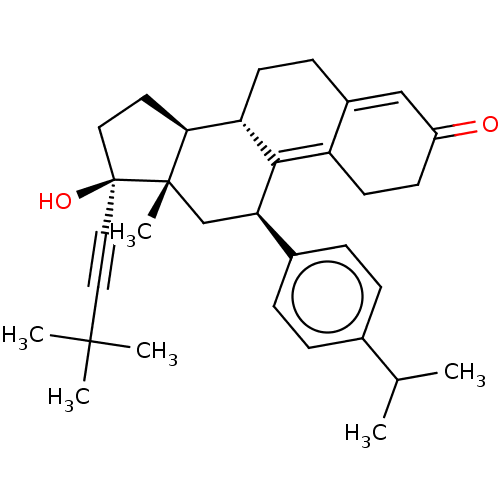 (CHEMBL4174418) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368948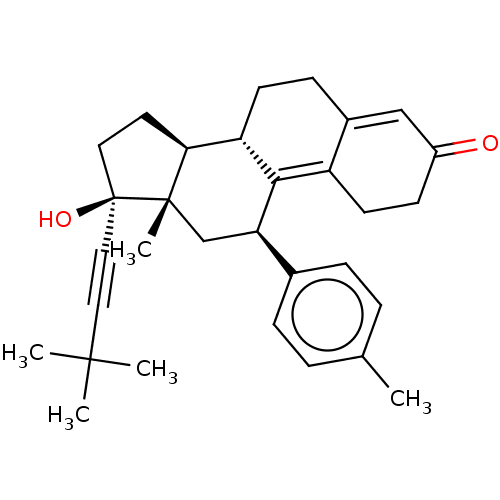 (CHEMBL4170986 | US11124537, TABLE 3.5.2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368932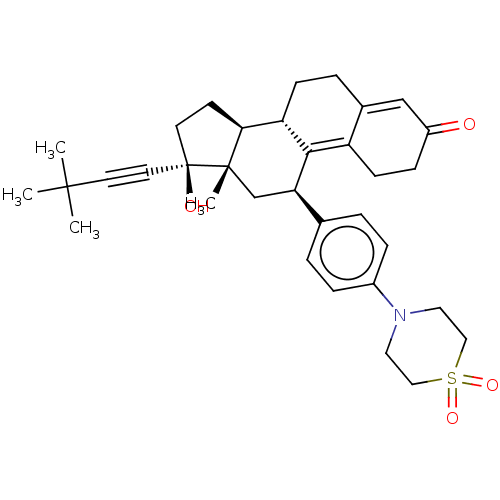 (CHEMBL4173222 | US11124537, TABLE 3.38.2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368933 (CHEMBL4161754) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in human OVCAR5 assessed as reduction in dexamethasone-induced GILZ gene expression incubated for 24 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368974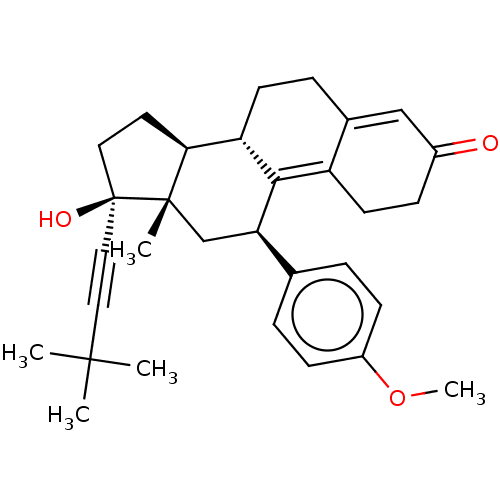 (CHEMBL4161175 | US11124537, TABLE 3.6.1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368935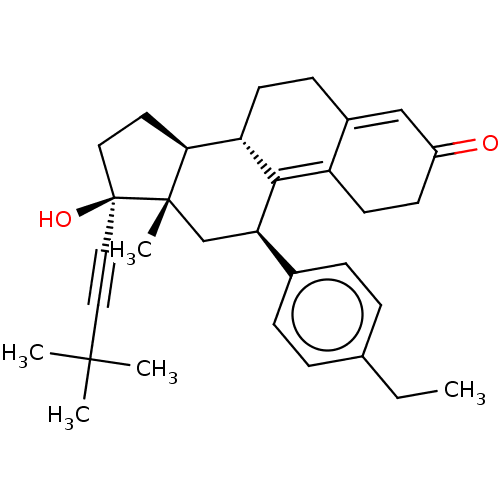 (CHEMBL4166499 | US11124537, TABLE 3.4.1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in human PBMC assessed as reduction in dexamethasone-induced GILZ gene expression incubated for 6 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368934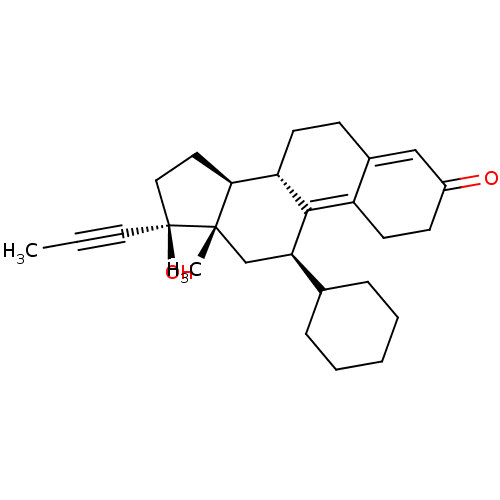 (CHEMBL4167332) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368938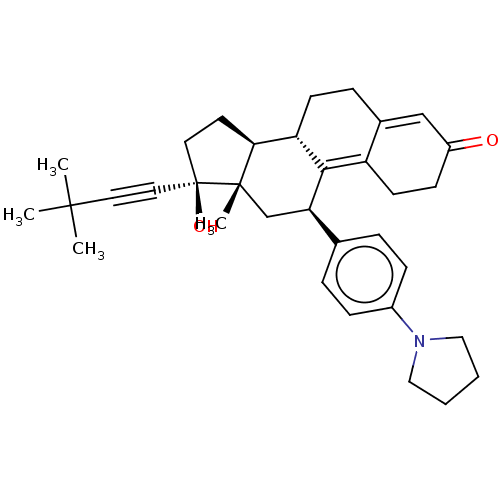 (CHEMBL4162539 | US11124537, TABLE 3.7.2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Canis lupus familiaris) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in dog PBMC assessed as reduction in dexamethasone-induced FKBP5 gene expression incubated for 6 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368933 (CHEMBL4161754) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in human PBMC assessed as reduction in dexamethasone-induced FKBP5 gene expression incubated for 6 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Sus scrofa) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in mini pig PBMC assessed as reduction in dexamethasone-induced FKBP5 gene expression incubated for 24 hrs by RT-qPCR metho... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368933 (CHEMBL4161754) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in human OVCAR5 assessed as reduction in dexamethasone-induced FKBP5 gene expression incubated for 24 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50368933 (CHEMBL4161754) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human PR expressed in CHO-K1 cells assessed as reduction in progesterone-induced response incubated for 20 hrs by luciferase r... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Canis lupus familiaris) | BDBM50368933 (CHEMBL4161754) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in dog PBMC assessed as reduction in dexamethasone-induced FKBP5 gene expression incubated for 6 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368933 (CHEMBL4161754) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in human PBMC assessed as reduction in dexamethasone-induced GILZ gene expression incubated for 6 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368978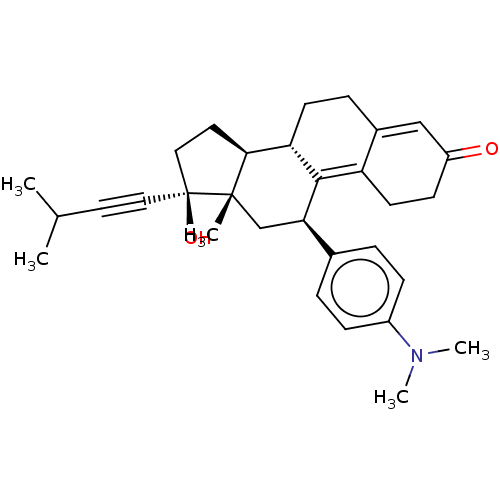 (CHEMBL4167608) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368973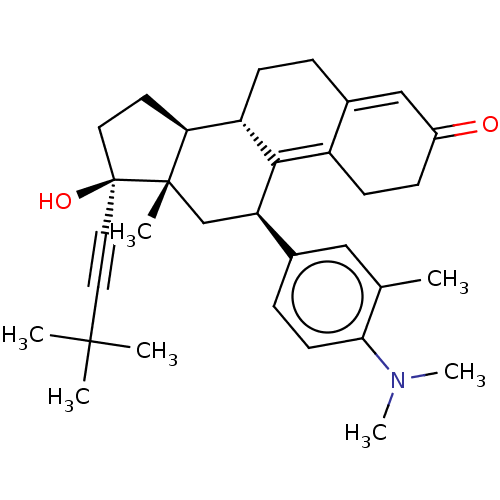 (CHEMBL4161483 | US11124537, TABLE 3.11.1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368963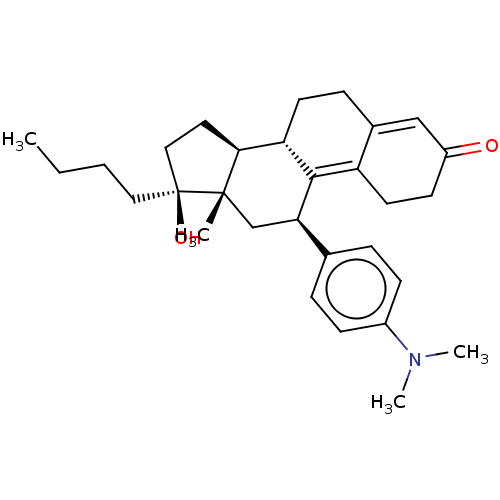 (CHEMBL4174812) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Canis lupus familiaris) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in dog PBMC assessed as reduction in dexamethasone-induced GILZ gene expression incubated for 6 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Canis lupus familiaris) | BDBM50368933 (CHEMBL4161754) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in dog PBMC assessed as reduction in dexamethasone-induced GILZ gene expression incubated for 6 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Sus scrofa) | BDBM50368933 (CHEMBL4161754) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in mini pig PBMC assessed as reduction in dexamethasone-induced FKBP5 gene expression incubated for 24 hrs by RT-qPCR metho... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368946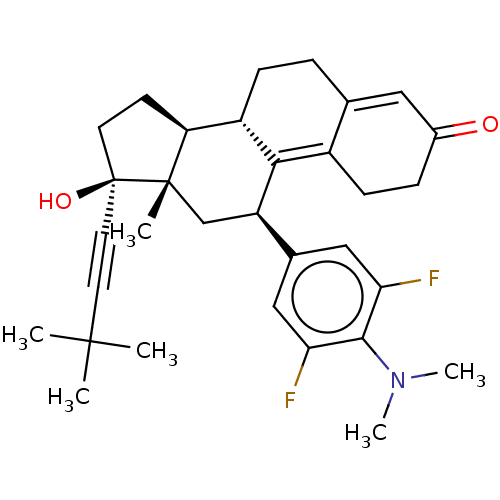 (CHEMBL4172798 | US11124537, TABLE 3.27.1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368972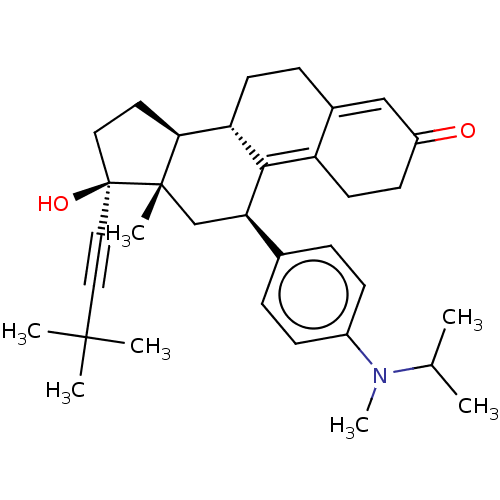 (CHEMBL4165975 | US11124537, TABLE 3.4.2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50368933 (CHEMBL4161754) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in rat PBMC assessed as reduction in dexamethasone-induced FKBP5 gene expression incubated for 24 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368942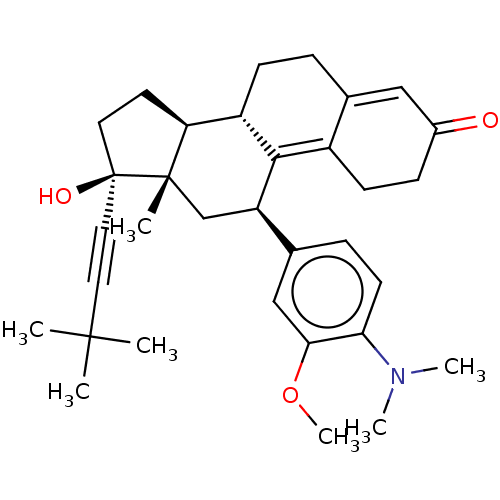 (CHEMBL4169408 | US11124537, TABLE 3.15.1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM50368933 (CHEMBL4161754) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in rat PBMC assessed as reduction in dexamethasone-induced GILZ gene expression incubated for 24 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368926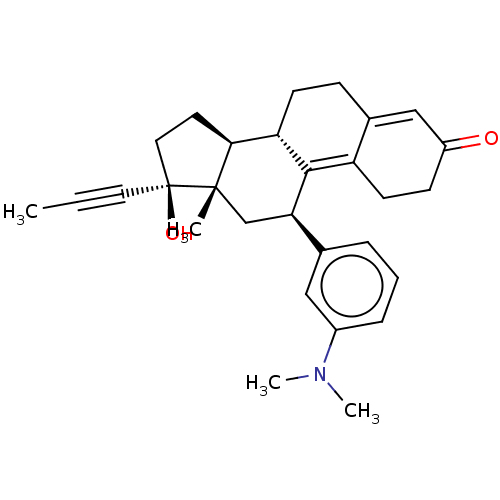 (CHEMBL4161968) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Sus scrofa) | BDBM50368933 (CHEMBL4161754) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at GR in mini pig PBMC assessed as reduction in dexamethasone-induced GILZ gene expression incubated for 24 hrs by RT-qPCR method | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50368965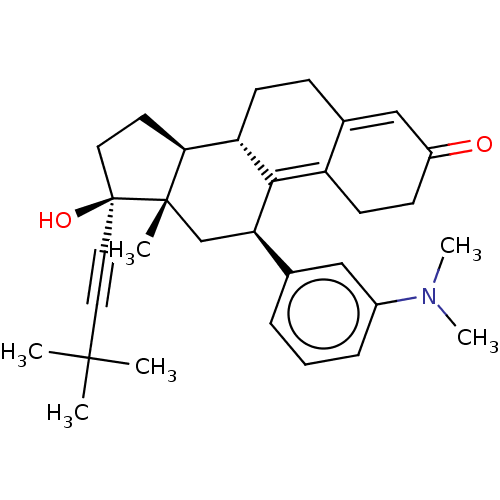 (CHEMBL4169100) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human GR expressed in CHO-K1 cells assessed as reduction in dexamethasone-induced response incubated for 20 hrs by luciferase ... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50368933 (CHEMBL4161754) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at AR (unknown origin) expressed in human LNCaP cells assessed as reduction in R1881-induced response incubated for 20 hrs by luc... | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) using midazolam as substrate | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50368933 (CHEMBL4161754) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using midazolam as substrate | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50368977 (CHEMBL4163069 | US11124537, TABLE 3.31.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ORIC Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) using midazolam as substrate | J Med Chem 61: 7767-7784 (2018) Article DOI: 10.1021/acs.jmedchem.8b00743 BindingDB Entry DOI: 10.7270/Q2C53PDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 113 total ) | Next | Last >> |