

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503761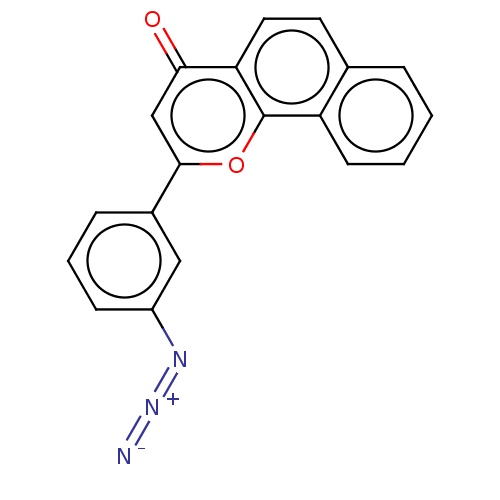 (CHEMBL4514232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503773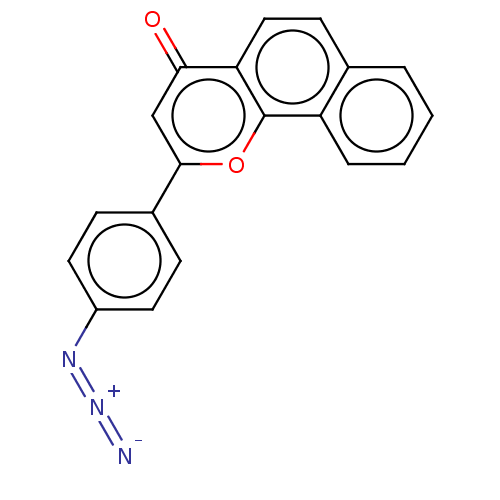 (CHEMBL4474660) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503774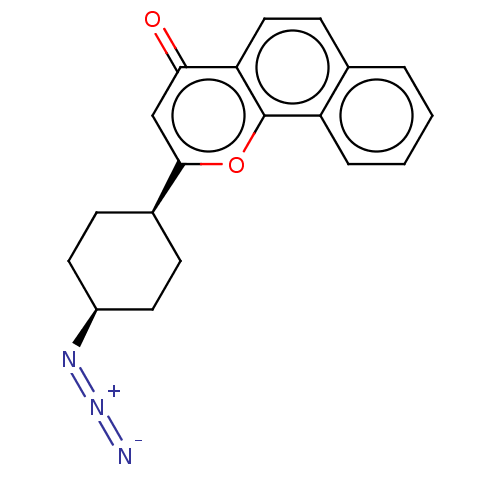 (CHEMBL4444783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50503773 (CHEMBL4474660) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503772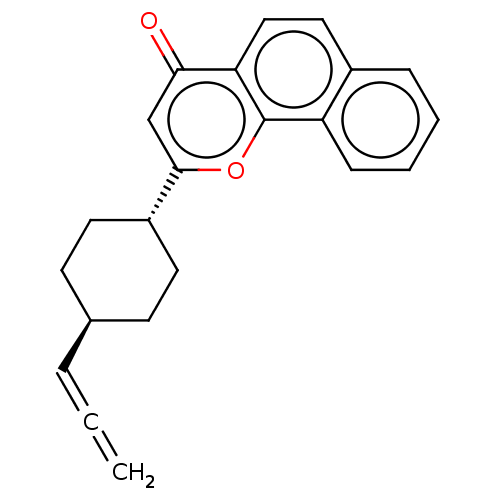 (CHEMBL4579760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50503772 (CHEMBL4579760) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50503767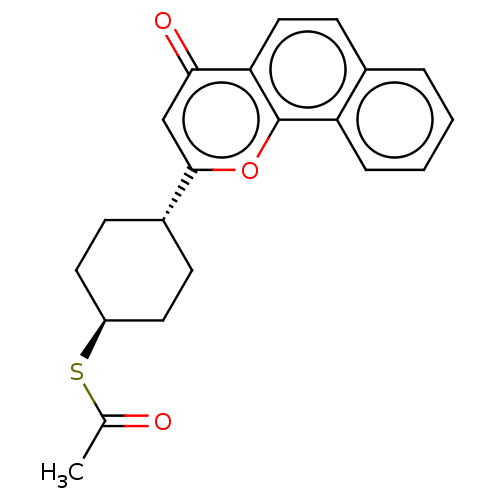 (CHEMBL4547387) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503770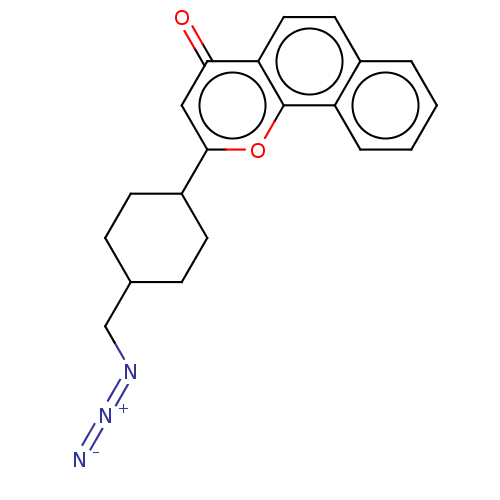 (CHEMBL4571646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503766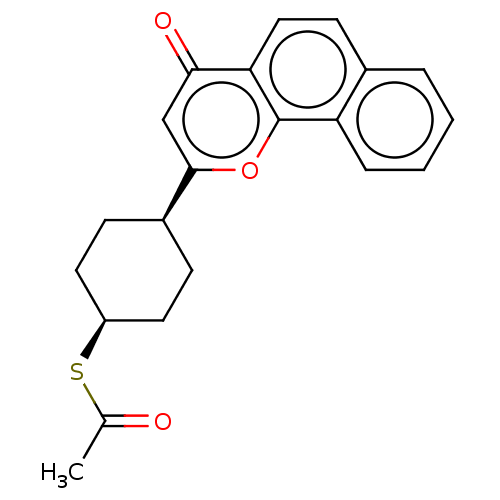 (CHEMBL4470607) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50503761 (CHEMBL4514232) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503767 (CHEMBL4547387) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50503769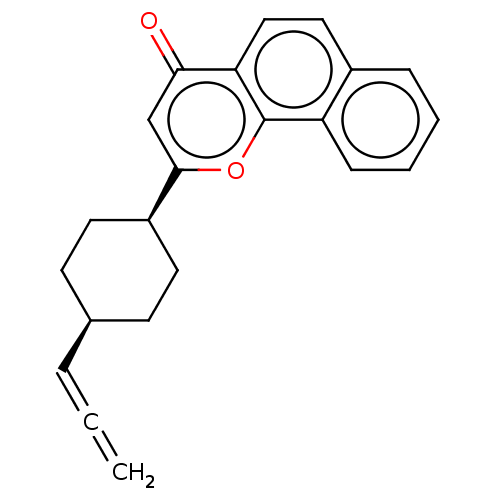 (CHEMBL4541853) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50503772 (CHEMBL4579760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503763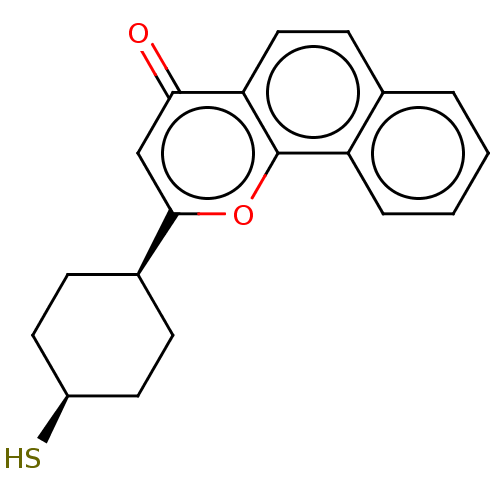 (CHEMBL4441141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503771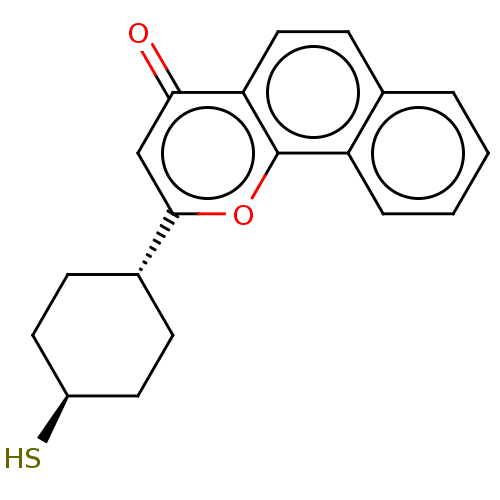 (CHEMBL4476231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM89011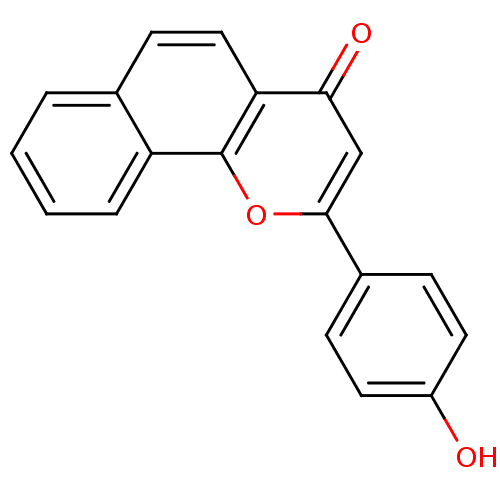 (2-(4-hydroxyphenyl)-4-benzo[h][1]benzopyranone | 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM89011 (2-(4-hydroxyphenyl)-4-benzo[h][1]benzopyranone | 2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50503766 (CHEMBL4470607) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503768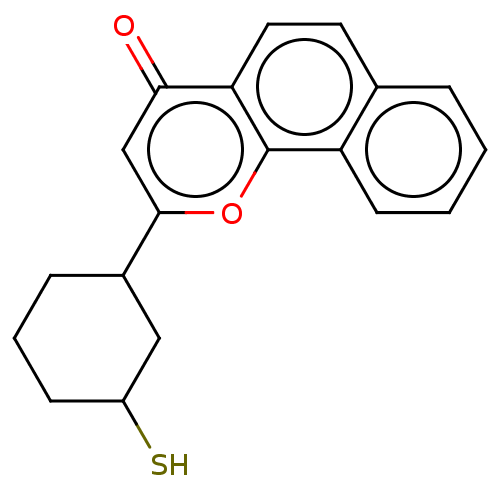 (CHEMBL4573324) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503769 (CHEMBL4541853) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50503761 (CHEMBL4514232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50503763 (CHEMBL4441141) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503762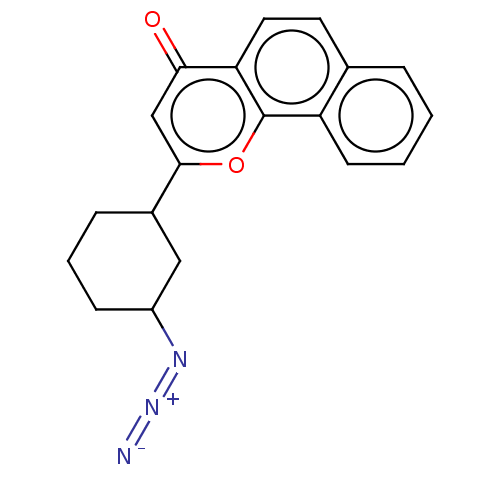 (CHEMBL4436010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50503773 (CHEMBL4474660) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50503768 (CHEMBL4573324) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503764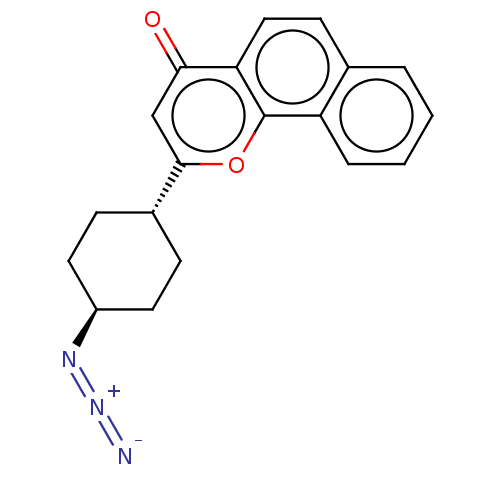 (CHEMBL4464279) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50503771 (CHEMBL4476231) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50503770 (CHEMBL4571646) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50503770 (CHEMBL4571646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 433 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50503774 (CHEMBL4444783) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 525 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50503765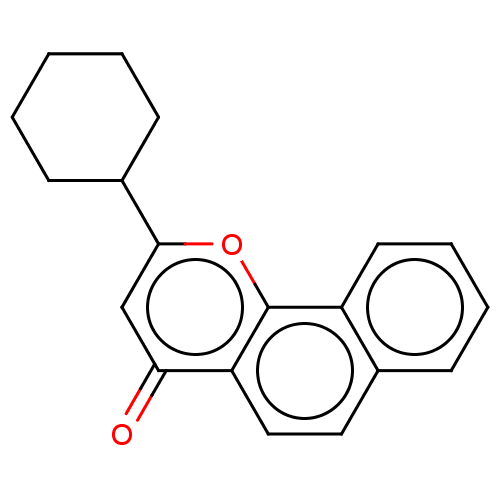 (CHEMBL1223487) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1B1 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50503774 (CHEMBL4444783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 667 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50503771 (CHEMBL4476231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 689 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM89011 (2-(4-hydroxyphenyl)-4-benzo[h][1]benzopyranone | 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 909 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50503767 (CHEMBL4547387) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 991 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50503764 (CHEMBL4464279) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A1 using 7-ethoxyresorufin as substrate after 15 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50503764 (CHEMBL4464279) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Showa Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate after 30 mins in presence of NADPH by EROD assay | Bioorg Med Chem 27: 285-304 (2019) Article DOI: 10.1016/j.bmc.2018.11.045 BindingDB Entry DOI: 10.7270/Q29C71NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||