

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at human GST-tagged estrogen receptor alpha ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at human GST-tagged estrogen receptor beta ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517935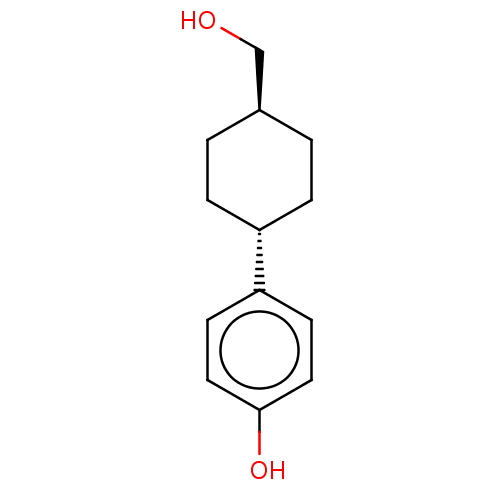 (CHEMBL4526434) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at human GST-tagged estrogen receptor beta ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517935 (CHEMBL4526434) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at human GST-tagged estrogen receptor alpha ligand binding domain assessed as coactivator peptide PGC1a recruitment by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor alpha (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50106635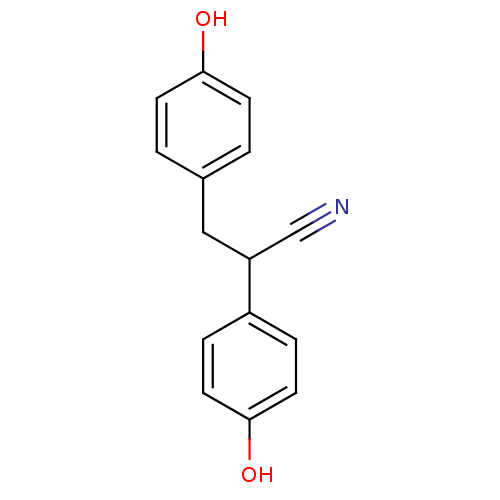 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517929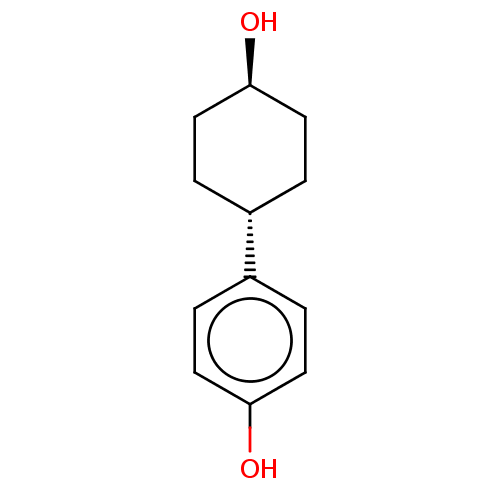 (CHEMBL4528491) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 7.52E+3 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517930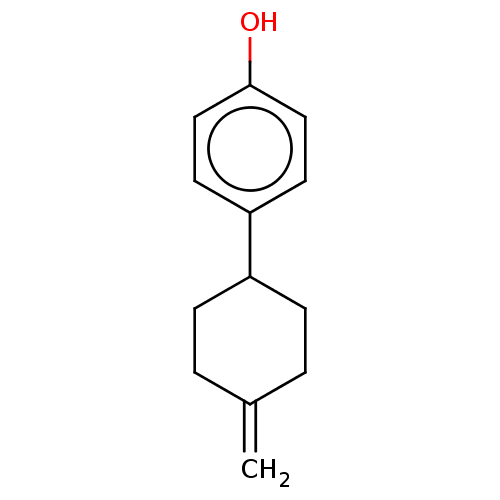 (CHEMBL4561540) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517931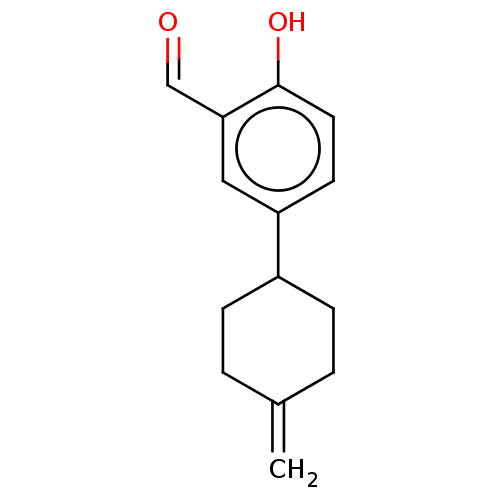 (CHEMBL4444171) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517932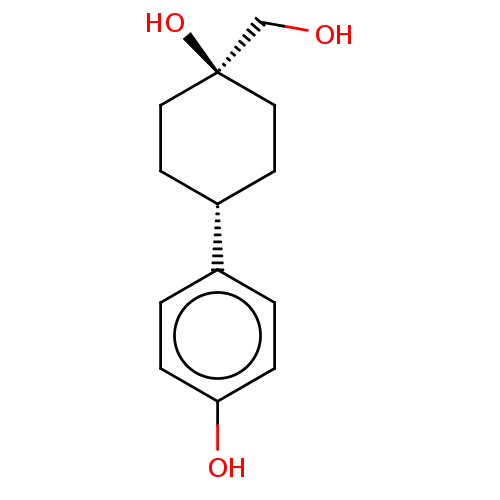 (CHEMBL4459147) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517933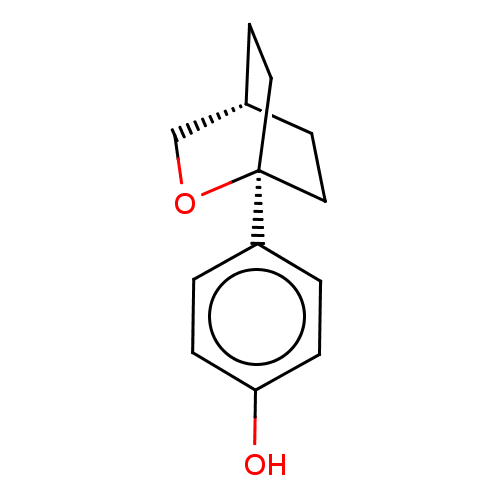 (CHEMBL4458536) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517928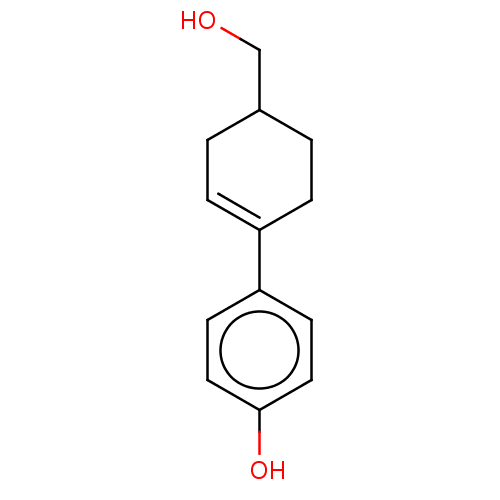 (CHEMBL4593988) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517934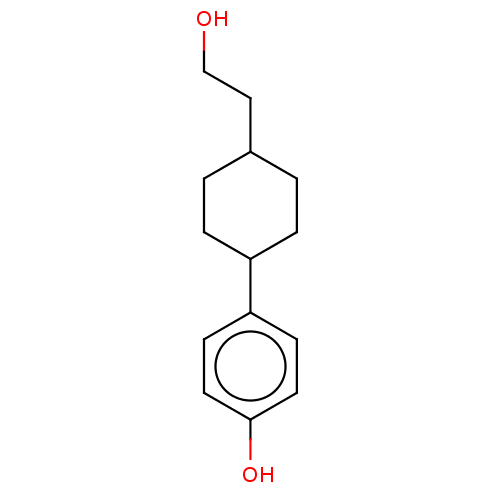 (CHEMBL4442761) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.151 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA binding domain fused full-length chimeric estrogen receptor beta (unknown origin) by FRET-based assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517935 (CHEMBL4526434) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 357 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA binding domain fused full-length chimeric estrogen receptor beta (unknown origin) by FRET-based assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517935 (CHEMBL4526434) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 930 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA binding domain fused full-length chimeric estrogen receptor alpha (unknown origin) by FRET-based assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50106635 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor beta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517930 (CHEMBL4561540) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 101 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor beta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517936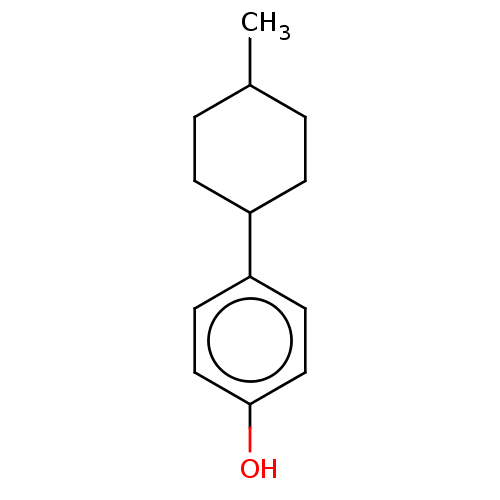 (CHEMBL4453541) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor beta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517928 (CHEMBL4593988) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor beta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517934 (CHEMBL4442761) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 75 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor beta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517930 (CHEMBL4561540) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor alpha (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517936 (CHEMBL4453541) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor alpha (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517928 (CHEMBL4593988) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor alpha (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50106635 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor beta (unknown origin) assessed as inhibition of estradiol-induced response after 22 hrs by cell-based lucife... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517930 (CHEMBL4561540) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor beta (unknown origin) assessed as inhibition of estradiol-induced response after 22 hrs by cell-based lucife... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517935 (CHEMBL4526434) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor beta (unknown origin) assessed as inhibition of estradiol-induced response after 22 hrs by cell-based lucife... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517928 (CHEMBL4593988) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor beta (unknown origin) assessed as inhibition of estradiol-induced response after 22 hrs by cell-based lucife... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50106635 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) assessed as inhibition of estradiol-induced response after 22 hrs by cell-based lucif... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517936 (CHEMBL4453541) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) assessed as inhibition of estradiol-induced response after 22 hrs by cell-based lucif... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517937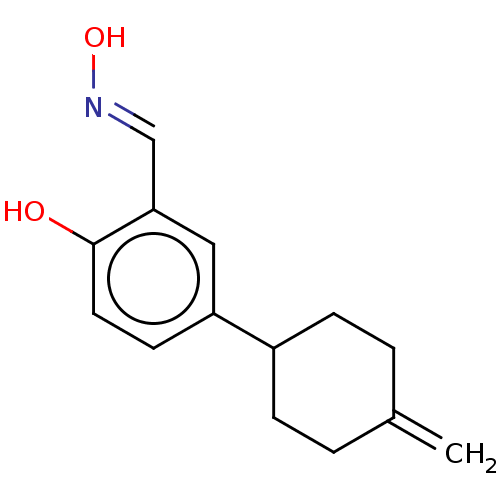 (CHEMBL4554635) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 225 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517935 (CHEMBL4526434) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 289 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor alpha ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50517935 (CHEMBL4526434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2C9 preincubated for 10 mins followed by NADPH addition and measured after 10 to 30 mins by P450-Glo luminescence ... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517935 (CHEMBL4526434) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) assessed as inhibition of estradiol-induced response after 22 hrs by cell-based lucif... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517934 (CHEMBL4442761) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor beta (unknown origin) assessed as inhibition of estradiol-induced response after 22 hrs by cell-based lucife... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50517935 (CHEMBL4526434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.90E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP3A4 preincubated for 10 mins followed by NADPH addition and measured after 10 to 30 mins by P450-Glo luminescence ... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517936 (CHEMBL4453541) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517935 (CHEMBL4526434) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor alpha (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.107 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA binding domain fused full-length chimeric estrogen receptor alpha (unknown origin) by FRET-based assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517935 (CHEMBL4526434) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor beta (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50106635 ((+/-)-2,3-bis(4-hydroxyphenyl)propanenitrile | 2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor alpha (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517934 (CHEMBL4442761) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at estrogen receptor alpha (unknown origin) after 22 hrs by cell-based luciferase reporter gene assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517936 (CHEMBL4453541) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor beta (unknown origin) assessed as inhibition of estradiol-induced response after 22 hrs by cell-based lucife... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517930 (CHEMBL4561540) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) assessed as inhibition of estradiol-induced response after 22 hrs by cell-based lucif... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517938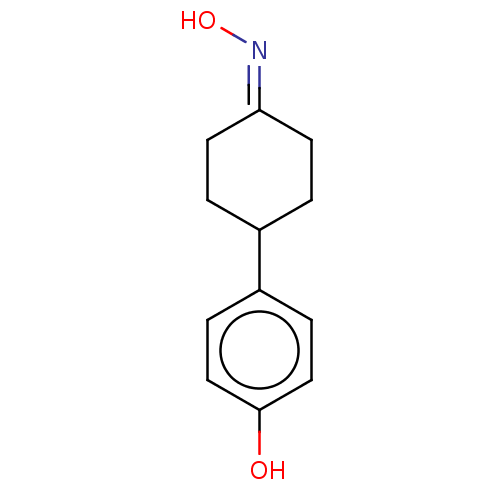 (CHEMBL4552891) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517935 (CHEMBL4526434) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50517939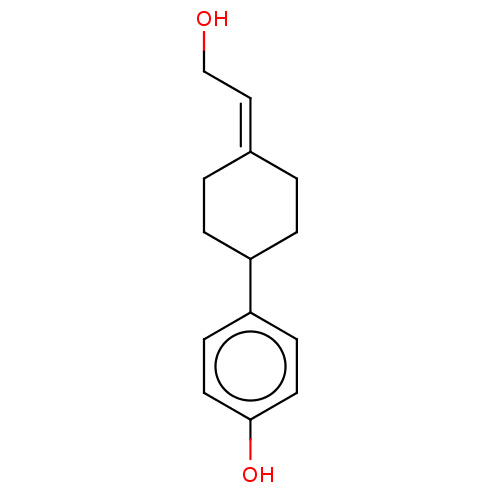 (CHEMBL4581589) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 680 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged estrogen receptor beta ligand binding domain after 1 hr by TR-FRET assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517934 (CHEMBL4442761) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) assessed as inhibition of estradiol-induced response after 22 hrs by cell-based lucif... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50517928 (CHEMBL4593988) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Antagonist activity at estrogen receptor alpha (unknown origin) assessed as inhibition of estradiol-induced response after 22 hrs by cell-based lucif... | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |