 Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50009102
Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50009102 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50525467
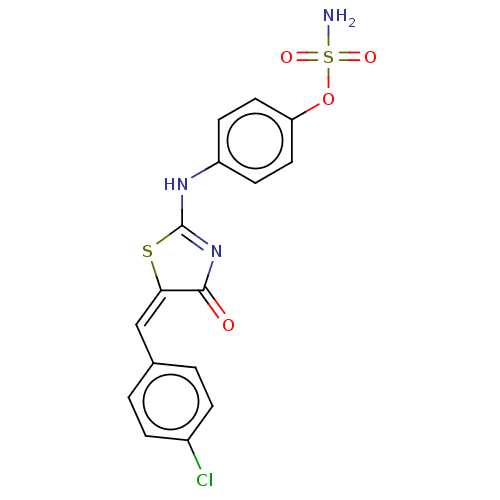
(CHEMBL4450307)Show SMILES NS(=O)(=O)Oc1ccc(NC2=NC(=O)\C(S2)=C/c2ccc(Cl)cc2)cc1 |t:10| Show InChI InChI=1S/C16H12ClN3O4S2/c17-11-3-1-10(2-4-11)9-14-15(21)20-16(25-14)19-12-5-7-13(8-6-12)24-26(18,22)23/h1-9H,(H2,18,22,23)(H,19,20,21)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50525475
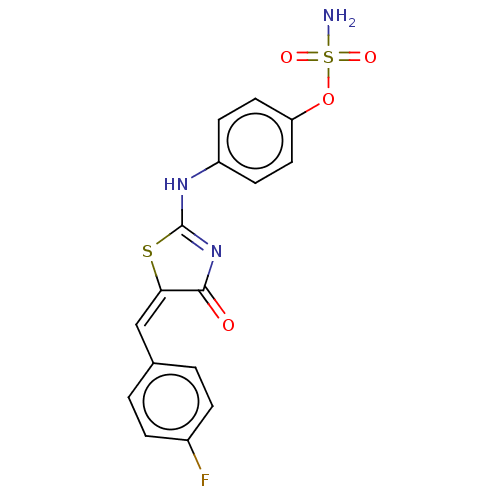
(CHEMBL4471122)Show SMILES NS(=O)(=O)Oc1ccc(NC2=NC(=O)\C(S2)=C/c2ccc(F)cc2)cc1 |t:10| Show InChI InChI=1S/C16H12FN3O4S2/c17-11-3-1-10(2-4-11)9-14-15(21)20-16(25-14)19-12-5-7-13(8-6-12)24-26(18,22)23/h1-9H,(H2,18,22,23)(H,19,20,21)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50525476
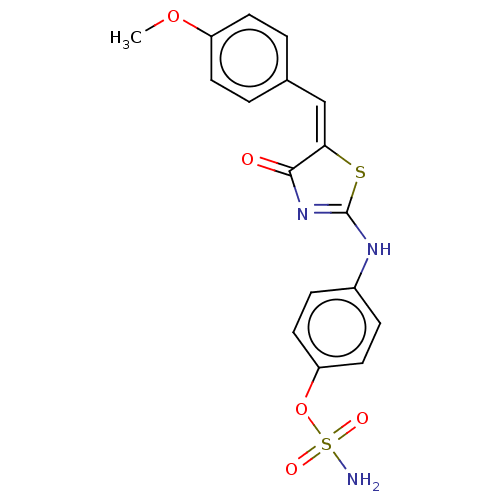
(CHEMBL4581749)Show SMILES COc1ccc(\C=C2\SC(Nc3ccc(OS(N)(=O)=O)cc3)=NC2=O)cc1 |c:22| Show InChI InChI=1S/C17H15N3O5S2/c1-24-13-6-2-11(3-7-13)10-15-16(21)20-17(26-15)19-12-4-8-14(9-5-12)25-27(18,22)23/h2-10H,1H3,(H2,18,22,23)(H,19,20,21)/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50525479
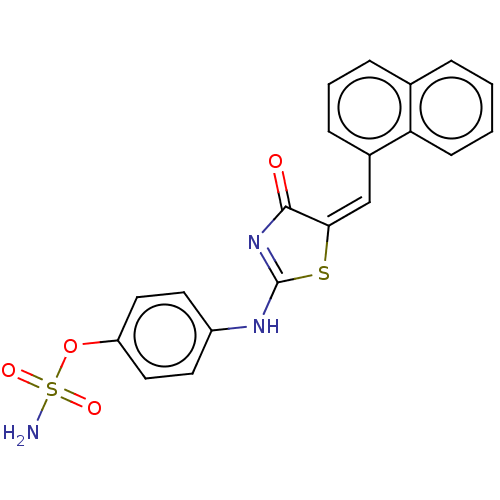
(CHEMBL4531097)Show SMILES NS(=O)(=O)Oc1ccc(NC2=NC(=O)\C(S2)=C/c2cccc3ccccc23)cc1 |t:10| Show InChI InChI=1S/C20H15N3O4S2/c21-29(25,26)27-16-10-8-15(9-11-16)22-20-23-19(24)18(28-20)12-14-6-3-5-13-4-1-2-7-17(13)14/h1-12H,(H2,21,25,26)(H,22,23,24)/b18-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50525477
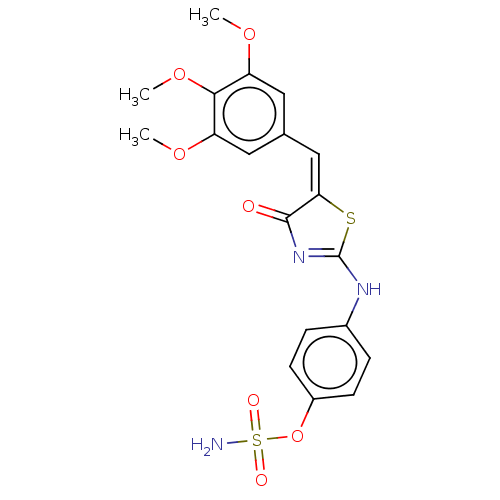
(CHEMBL4554643)Show SMILES COc1cc(\C=C2\SC(Nc3ccc(OS(N)(=O)=O)cc3)=NC2=O)cc(OC)c1OC |c:21| Show InChI InChI=1S/C19H19N3O7S2/c1-26-14-8-11(9-15(27-2)17(14)28-3)10-16-18(23)22-19(30-16)21-12-4-6-13(7-5-12)29-31(20,24)25/h4-10H,1-3H3,(H2,20,24,25)(H,21,22,23)/b16-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50525478
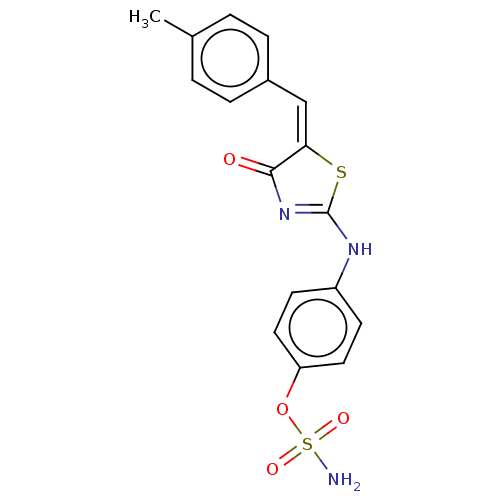
(CHEMBL4533212)Show SMILES Cc1ccc(\C=C2\SC(Nc3ccc(OS(N)(=O)=O)cc3)=NC2=O)cc1 |c:21| Show InChI InChI=1S/C17H15N3O4S2/c1-11-2-4-12(5-3-11)10-15-16(21)20-17(25-15)19-13-6-8-14(9-7-13)24-26(18,22)23/h2-10H,1H3,(H2,18,22,23)(H,19,20,21)/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50525466
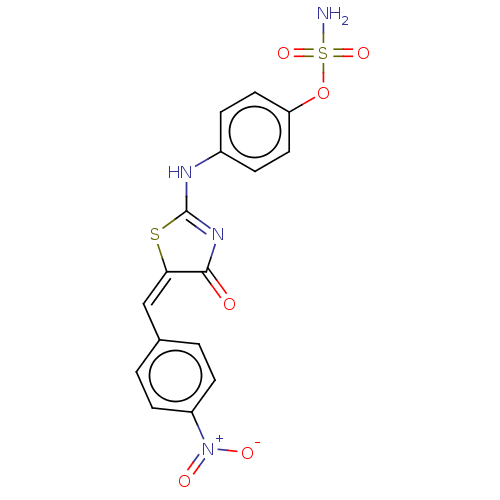
(CHEMBL4440045)Show SMILES NS(=O)(=O)Oc1ccc(NC2=NC(=O)\C(S2)=C/c2ccc(cc2)[N+]([O-])=O)cc1 |t:10| Show InChI InChI=1S/C16H12N4O6S2/c17-28(24,25)26-13-7-3-11(4-8-13)18-16-19-15(21)14(27-16)9-10-1-5-12(6-2-10)20(22)23/h1-9H,(H2,17,24,25)(H,18,19,21)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50525474
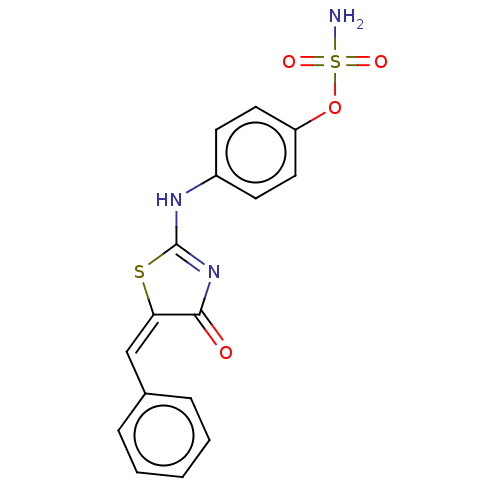
(CHEMBL4457432)Show SMILES NS(=O)(=O)Oc1ccc(NC2=NC(=O)\C(S2)=C/c2ccccc2)cc1 |t:10| Show InChI InChI=1S/C16H13N3O4S2/c17-25(21,22)23-13-8-6-12(7-9-13)18-16-19-15(20)14(24-16)10-11-4-2-1-3-5-11/h1-10H,(H2,17,21,22)(H,18,19,20)/b14-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50525483
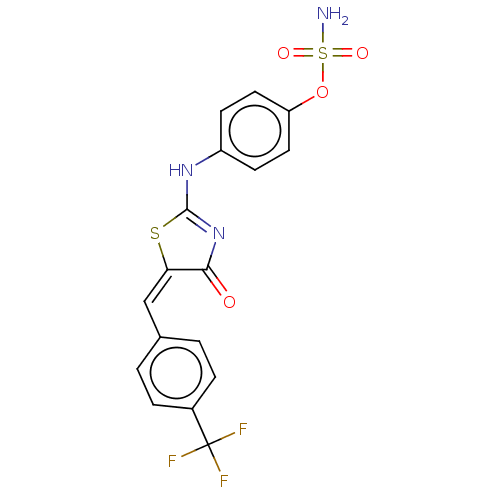
(CHEMBL4546356)Show SMILES NS(=O)(=O)Oc1ccc(NC2=NC(=O)\C(S2)=C/c2ccc(cc2)C(F)(F)F)cc1 |t:10| Show InChI InChI=1S/C17H12F3N3O4S2/c18-17(19,20)11-3-1-10(2-4-11)9-14-15(24)23-16(28-14)22-12-5-7-13(8-6-12)27-29(21,25)26/h1-9H,(H2,21,25,26)(H,22,23,24)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50525474

(CHEMBL4457432)Show SMILES NS(=O)(=O)Oc1ccc(NC2=NC(=O)\C(S2)=C/c2ccccc2)cc1 |t:10| Show InChI InChI=1S/C16H13N3O4S2/c17-25(21,22)23-13-8-6-12(7-9-13)18-16-19-15(20)14(24-16)10-11-4-2-1-3-5-11/h1-10H,(H2,17,21,22)(H,18,19,20)/b14-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50525479

(CHEMBL4531097)Show SMILES NS(=O)(=O)Oc1ccc(NC2=NC(=O)\C(S2)=C/c2cccc3ccccc23)cc1 |t:10| Show InChI InChI=1S/C20H15N3O4S2/c21-29(25,26)27-16-10-8-15(9-11-16)22-20-23-19(24)18(28-20)12-14-6-3-5-13-4-1-2-7-17(13)14/h1-12H,(H2,21,25,26)(H,22,23,24)/b18-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50525477

(CHEMBL4554643)Show SMILES COc1cc(\C=C2\SC(Nc3ccc(OS(N)(=O)=O)cc3)=NC2=O)cc(OC)c1OC |c:21| Show InChI InChI=1S/C19H19N3O7S2/c1-26-14-8-11(9-15(27-2)17(14)28-3)10-16-18(23)22-19(30-16)21-12-4-6-13(7-5-12)29-31(20,24)25/h4-10H,1-3H3,(H2,20,24,25)(H,21,22,23)/b16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50525476

(CHEMBL4581749)Show SMILES COc1ccc(\C=C2\SC(Nc3ccc(OS(N)(=O)=O)cc3)=NC2=O)cc1 |c:22| Show InChI InChI=1S/C17H15N3O5S2/c1-24-13-6-2-11(3-7-13)10-15-16(21)20-17(26-15)19-12-4-8-14(9-5-12)25-27(18,22)23/h2-10H,1H3,(H2,18,22,23)(H,19,20,21)/b15-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50525466

(CHEMBL4440045)Show SMILES NS(=O)(=O)Oc1ccc(NC2=NC(=O)\C(S2)=C/c2ccc(cc2)[N+]([O-])=O)cc1 |t:10| Show InChI InChI=1S/C16H12N4O6S2/c17-28(24,25)26-13-7-3-11(4-8-13)18-16-19-15(21)14(27-16)9-10-1-5-12(6-2-10)20(22)23/h1-9H,(H2,17,24,25)(H,18,19,21)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50525483

(CHEMBL4546356)Show SMILES NS(=O)(=O)Oc1ccc(NC2=NC(=O)\C(S2)=C/c2ccc(cc2)C(F)(F)F)cc1 |t:10| Show InChI InChI=1S/C17H12F3N3O4S2/c18-17(19,20)11-3-1-10(2-4-11)9-14-15(24)23-16(28-14)22-12-5-7-13(8-6-12)27-29(21,25)26/h1-9H,(H2,21,25,26)(H,22,23,24)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50525478

(CHEMBL4533212)Show SMILES Cc1ccc(\C=C2\SC(Nc3ccc(OS(N)(=O)=O)cc3)=NC2=O)cc1 |c:21| Show InChI InChI=1S/C17H15N3O4S2/c1-11-2-4-12(5-3-11)10-15-16(21)20-17(25-15)19-13-6-8-14(9-7-13)24-26(18,22)23/h2-10H,1H3,(H2,18,22,23)(H,19,20,21)/b15-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50525467

(CHEMBL4450307)Show SMILES NS(=O)(=O)Oc1ccc(NC2=NC(=O)\C(S2)=C/c2ccc(Cl)cc2)cc1 |t:10| Show InChI InChI=1S/C16H12ClN3O4S2/c17-11-3-1-10(2-4-11)9-14-15(21)20-16(25-14)19-12-5-7-13(8-6-12)24-26(18,22)23/h1-9H,(H2,18,22,23)(H,19,20,21)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50525475

(CHEMBL4471122)Show SMILES NS(=O)(=O)Oc1ccc(NC2=NC(=O)\C(S2)=C/c2ccc(F)cc2)cc1 |t:10| Show InChI InChI=1S/C16H12FN3O4S2/c17-11-3-1-10(2-4-11)9-14-15(21)20-16(25-14)19-12-5-7-13(8-6-12)24-26(18,22)23/h1-9H,(H2,18,22,23)(H,19,20,21)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50525477

(CHEMBL4554643)Show SMILES COc1cc(\C=C2\SC(Nc3ccc(OS(N)(=O)=O)cc3)=NC2=O)cc(OC)c1OC |c:21| Show InChI InChI=1S/C19H19N3O7S2/c1-26-14-8-11(9-15(27-2)17(14)28-3)10-16-18(23)22-19(30-16)21-12-4-6-13(7-5-12)29-31(20,24)25/h4-10H,1-3H3,(H2,20,24,25)(H,21,22,23)/b16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50525474

(CHEMBL4457432)Show SMILES NS(=O)(=O)Oc1ccc(NC2=NC(=O)\C(S2)=C/c2ccccc2)cc1 |t:10| Show InChI InChI=1S/C16H13N3O4S2/c17-25(21,22)23-13-8-6-12(7-9-13)18-16-19-15(20)14(24-16)10-11-4-2-1-3-5-11/h1-10H,(H2,17,21,22)(H,18,19,20)/b14-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 4 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50136297
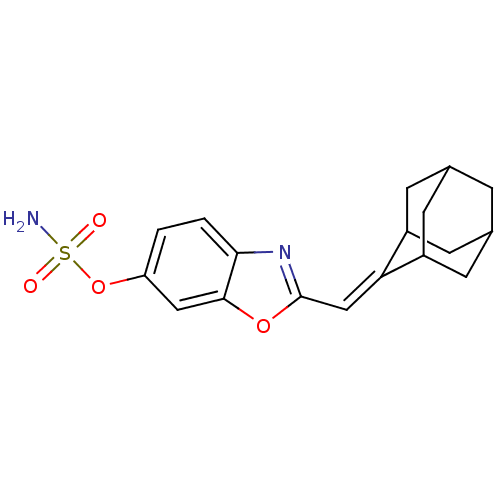
(CHEMBL136112 | Sulfamic acid 2-adamantan-2-ylidene...)Show SMILES NS(=O)(=O)Oc1ccc2nc(C=C3C4CC5CC(C4)CC3C5)oc2c1 |TLB:21:20:18:15.14.16,THB:21:15:12.20.19:18,16:15:12:17.19.18,16:17:12:15.14.21,11:12:18:15.14.16,(-1.25,-4.19,;.08,-3.42,;-.69,-2.08,;.85,-2.08,;1.41,-4.19,;2.76,-3.42,;2.76,-1.87,;4.09,-1.1,;5.42,-1.86,;6.91,-1.38,;7.82,-2.63,;9.36,-2.63,;10.13,-1.29,;9.45,.09,;10.55,1.03,;11.96,1.18,;12.47,2.87,;11.3,1.82,;9.8,1.7,;12.03,.4,;11.65,-1.18,;12.63,-.14,;6.91,-3.9,;5.42,-3.42,;4.09,-4.19,)| Show InChI InChI=1S/C18H20N2O4S/c19-25(21,22)24-14-1-2-16-17(8-14)23-18(20-16)9-15-12-4-10-3-11(6-12)7-13(15)5-10/h1-2,8-13H,3-7H2,(H2,19,21,22)/b15-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) expressed in CHO cells |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50136297

(CHEMBL136112 | Sulfamic acid 2-adamantan-2-ylidene...)Show SMILES NS(=O)(=O)Oc1ccc2nc(C=C3C4CC5CC(C4)CC3C5)oc2c1 |TLB:21:20:18:15.14.16,THB:21:15:12.20.19:18,16:15:12:17.19.18,16:17:12:15.14.21,11:12:18:15.14.16,(-1.25,-4.19,;.08,-3.42,;-.69,-2.08,;.85,-2.08,;1.41,-4.19,;2.76,-3.42,;2.76,-1.87,;4.09,-1.1,;5.42,-1.86,;6.91,-1.38,;7.82,-2.63,;9.36,-2.63,;10.13,-1.29,;9.45,.09,;10.55,1.03,;11.96,1.18,;12.47,2.87,;11.3,1.82,;9.8,1.7,;12.03,.4,;11.65,-1.18,;12.63,-.14,;6.91,-3.9,;5.42,-3.42,;4.09,-4.19,)| Show InChI InChI=1S/C18H20N2O4S/c19-25(21,22)24-14-1-2-16-17(8-14)23-18(20-16)9-15-12-4-10-3-11(6-12)7-13(15)5-10/h1-2,8-13H,3-7H2,(H2,19,21,22)/b15-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) expressed in human MCF7 cells |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50121079

(CHEMBL3622064)Show SMILES NS(=O)(=O)Oc1ccc2cc(CN(c3ccc(cc3)C#N)n3cnnc3)ccc2c1Br Show InChI InChI=1S/C20H15BrN6O3S/c21-20-18-7-3-15(9-16(18)4-8-19(20)30-31(23,28)29)11-27(26-12-24-25-13-26)17-5-1-14(10-22)2-6-17/h1-9,12-13H,11H2,(H2,23,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118560
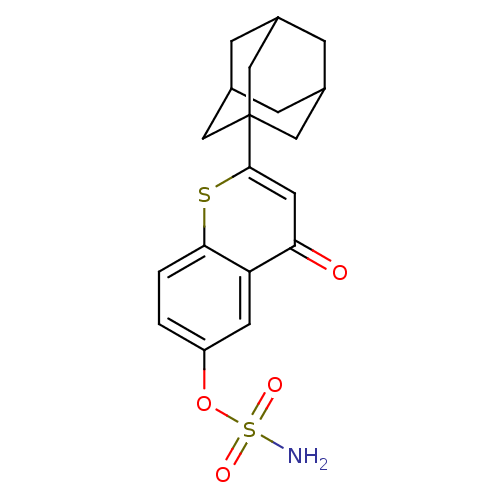
(CHEMBL262050 | Sulfamic acid 2-adamantan-1-yl-4-ox...)Show SMILES NS(=O)(=O)Oc1ccc2sc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:23:22:25:17.18.19,23:18:25:24.22.21,THB:21:22:17:25.20.19,21:20:17:24.22.23| Show InChI InChI=1S/C19H21NO4S2/c20-26(22,23)24-14-1-2-17-15(6-14)16(21)7-18(25-17)19-8-11-3-12(9-19)5-13(4-11)10-19/h1-2,6-7,11-13H,3-5,8-10H2,(H2,20,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human steroid sulfatase by fluorimetric assay |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525470

(CHEMBL4456330)Show SMILES CCCCCCC(O)(CCCCc1ccc(OS(N)(=O)=O)cc1)c1ccc(cc1)C(C)(C)C Show InChI InChI=1S/C27H41NO4S/c1-5-6-7-9-20-27(29,24-16-14-23(15-17-24)26(2,3)4)21-10-8-11-22-12-18-25(19-13-22)32-33(28,30)31/h12-19,29H,5-11,20-21H2,1-4H3,(H2,28,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human placental steroid sulfatase expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by liquid scintillation counting me... |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525473
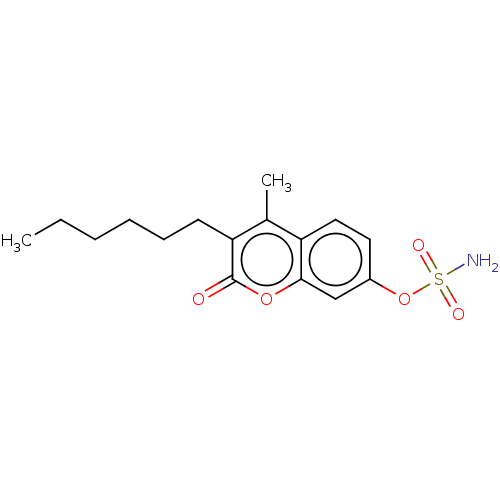
(CHEMBL4520442)Show InChI InChI=1S/C16H21NO5S/c1-3-4-5-6-7-14-11(2)13-9-8-12(22-23(17,19)20)10-15(13)21-16(14)18/h8-10H,3-7H2,1-2H3,(H2,17,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human MCF7 cells |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human placental steroid sulfatase expressed in HEK293 cells using [3H] E1S as substrate after 2 hrs by liquid scintillation counting me... |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525481
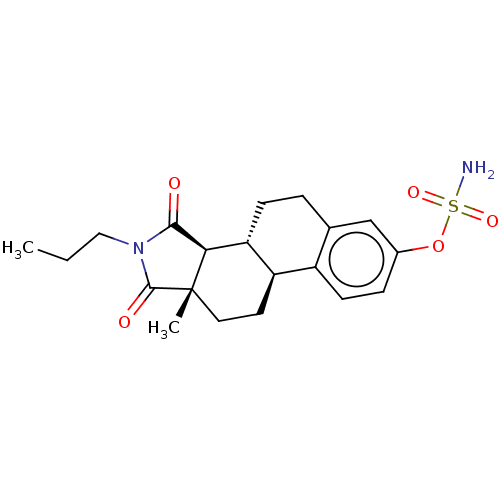
(CHEMBL4436186)Show SMILES [H][C@@]12C(=O)N(CCC)C(=O)[C@@]1(C)CC[C@]1([H])c3ccc(OS(N)(=O)=O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C20H26N2O5S/c1-3-10-22-18(23)17-16-6-4-12-11-13(27-28(21,25)26)5-7-14(12)15(16)8-9-20(17,2)19(22)24/h5,7,11,15-17H,3-4,6,8-10H2,1-2H3,(H2,21,25,26)/t15-,16-,17-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525472
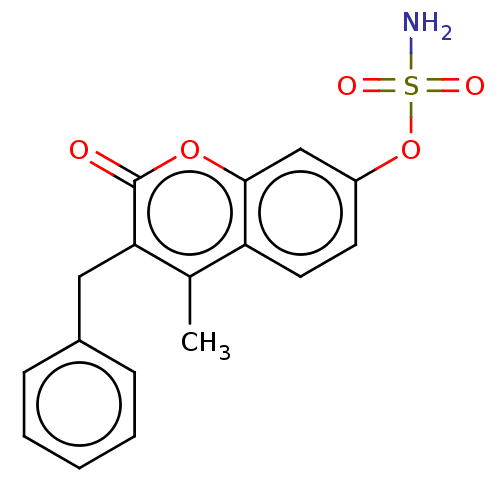
(CHEMBL4459312)Show SMILES Cc1c(Cc2ccccc2)c(=O)oc2cc(OS(N)(=O)=O)ccc12 Show InChI InChI=1S/C17H15NO5S/c1-11-14-8-7-13(23-24(18,20)21)10-16(14)22-17(19)15(11)9-12-5-3-2-4-6-12/h2-8,10H,9H2,1H3,(H2,18,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human MCF7 cells |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50121063
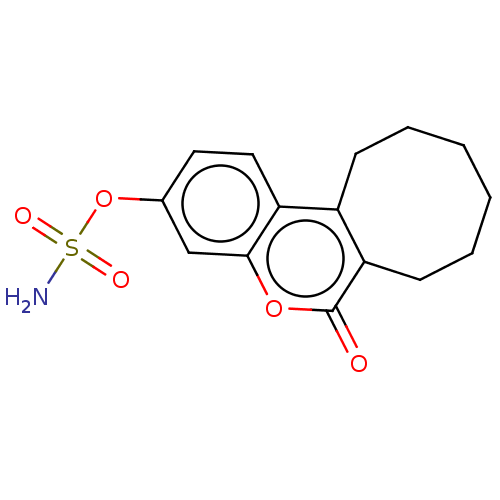
(CHEMBL3622010)Show InChI InChI=1S/C15H17NO5S/c16-22(18,19)21-10-7-8-12-11-5-3-1-2-4-6-13(11)15(17)20-14(12)9-10/h7-9H,1-6H2,(H2,16,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525469
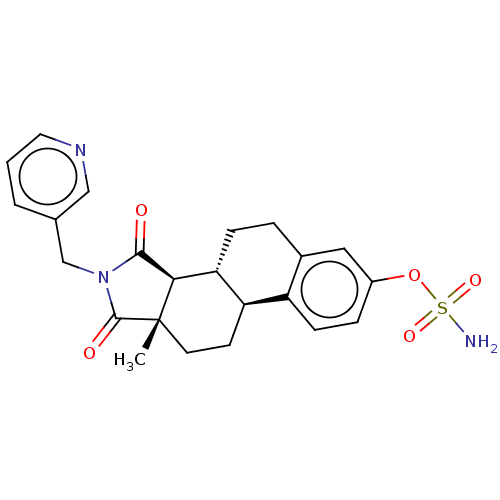
(CHEMBL4517286)Show SMILES [H][C@@]12C(=O)N(Cc3cccnc3)C(=O)[C@@]1(C)CC[C@]1([H])c3ccc(OS(N)(=O)=O)cc3CC[C@@]21[H] |r| Show InChI InChI=1S/C23H25N3O5S/c1-23-9-8-18-17-7-5-16(31-32(24,29)30)11-15(17)4-6-19(18)20(23)21(27)26(22(23)28)13-14-3-2-10-25-12-14/h2-3,5,7,10-12,18-20H,4,6,8-9,13H2,1H3,(H2,24,29,30)/t18-,19-,20-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525471
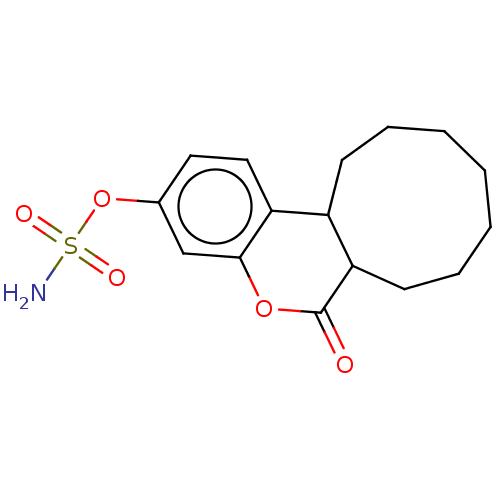
(CHEMBL4442535)Show InChI InChI=1S/C16H21NO5S/c17-23(19,20)22-11-8-9-13-12-6-4-2-1-3-5-7-14(12)16(18)21-15(13)10-11/h8-10,12,14H,1-7H2,(H2,17,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525472

(CHEMBL4459312)Show SMILES Cc1c(Cc2ccccc2)c(=O)oc2cc(OS(N)(=O)=O)ccc12 Show InChI InChI=1S/C17H15NO5S/c1-11-14-8-7-13(23-24(18,20)21)10-16(14)22-17(19)15(11)9-12-5-3-2-4-6-12/h2-8,10H,9H2,1H3,(H2,18,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase derived form human placental microsomes |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50525468
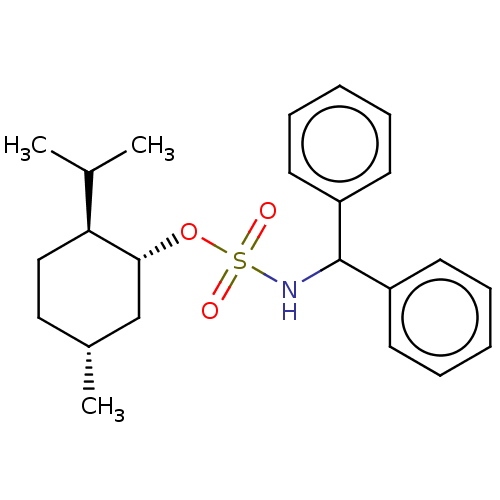
(CHEMBL4463022)Show SMILES CC(C)[C@@H]1CC[C@@H](C)C[C@H]1OS(=O)(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C23H31NO3S/c1-17(2)21-15-14-18(3)16-22(21)27-28(25,26)24-23(19-10-6-4-7-11-19)20-12-8-5-9-13-20/h4-13,17-18,21-24H,14-16H2,1-3H3/t18-,21+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using p-nitrophenylacetate as substrate after 3 mins |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50121078
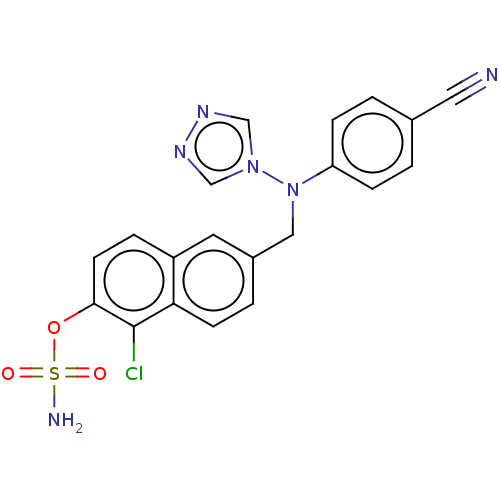
(CHEMBL3622063)Show SMILES NS(=O)(=O)Oc1ccc2cc(CN(c3ccc(cc3)C#N)n3cnnc3)ccc2c1Cl Show InChI InChI=1S/C20H15ClN6O3S/c21-20-18-7-3-15(9-16(18)4-8-19(20)30-31(23,28)29)11-27(26-12-24-25-13-26)17-5-1-14(10-22)2-6-17/h1-9,12-13H,11H2,(H2,23,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50051829
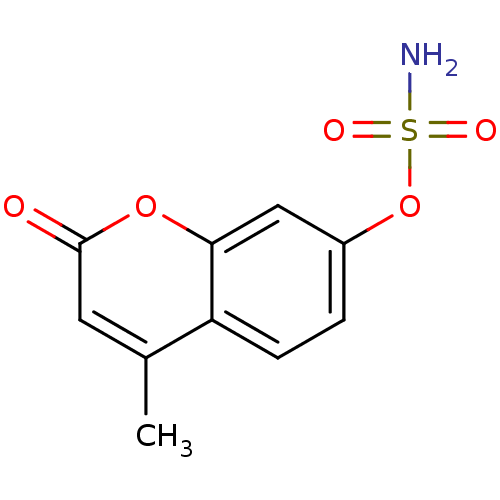
(4-methyl-2-oxo-2H-chromen-7-yl sulfamate | CHEMBL1...)Show InChI InChI=1S/C10H9NO5S/c1-6-4-10(12)15-9-5-7(2-3-8(6)9)16-17(11,13)14/h2-5H,1H3,(H2,11,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525473

(CHEMBL4520442)Show InChI InChI=1S/C16H21NO5S/c1-3-4-5-6-7-14-11(2)13-9-8-12(22-23(17,19)20)10-15(13)21-16(14)18/h8-10H,3-7H2,1-2H3,(H2,17,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase derived form human placental microsomes |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50525468

(CHEMBL4463022)Show SMILES CC(C)[C@@H]1CC[C@@H](C)C[C@H]1OS(=O)(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C23H31NO3S/c1-17(2)21-15-14-18(3)16-22(21)27-28(25,26)24-23(19-10-6-4-7-11-19)20-12-8-5-9-13-20/h4-13,17-18,21-24H,14-16H2,1-3H3/t18-,21+,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using p-nitrophenylacetate as substrate after 3 mins |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50525482
(CHEMBL4547200)Show SMILES NS(=O)(=O)Oc1ccc(\C=C\C(=O)C[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C16H21NO9S/c17-27(23,24)26-11-5-2-9(3-6-11)1-4-10(19)7-12-14(20)16(22)15(21)13(8-18)25-12/h1-6,12-16,18,20-22H,7-8H2,(H2,17,23,24)/b4-1+/t12-,13+,14-,15+,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50525468

(CHEMBL4463022)Show SMILES CC(C)[C@@H]1CC[C@@H](C)C[C@H]1OS(=O)(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C23H31NO3S/c1-17(2)21-15-14-18(3)16-22(21)27-28(25,26)24-23(19-10-6-4-7-11-19)20-12-8-5-9-13-20/h4-13,17-18,21-24H,14-16H2,1-3H3/t18-,21+,22-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of electric ee AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by Ellman's method |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50525468

(CHEMBL4463022)Show SMILES CC(C)[C@@H]1CC[C@@H](C)C[C@H]1OS(=O)(=O)NC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C23H31NO3S/c1-17(2)21-15-14-18(3)16-22(21)27-28(25,26)24-23(19-10-6-4-7-11-19)20-12-8-5-9-13-20/h4-13,17-18,21-24H,14-16H2,1-3H3/t18-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of equine BChE using butyrylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by Ellman's method |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50136297

(CHEMBL136112 | Sulfamic acid 2-adamantan-2-ylidene...)Show SMILES NS(=O)(=O)Oc1ccc2nc(C=C3C4CC5CC(C4)CC3C5)oc2c1 |TLB:21:20:18:15.14.16,THB:21:15:12.20.19:18,16:15:12:17.19.18,16:17:12:15.14.21,11:12:18:15.14.16,(-1.25,-4.19,;.08,-3.42,;-.69,-2.08,;.85,-2.08,;1.41,-4.19,;2.76,-3.42,;2.76,-1.87,;4.09,-1.1,;5.42,-1.86,;6.91,-1.38,;7.82,-2.63,;9.36,-2.63,;10.13,-1.29,;9.45,.09,;10.55,1.03,;11.96,1.18,;12.47,2.87,;11.3,1.82,;9.8,1.7,;12.03,.4,;11.65,-1.18,;12.63,-.14,;6.91,-3.9,;5.42,-3.42,;4.09,-4.19,)| Show InChI InChI=1S/C18H20N2O4S/c19-25(21,22)24-14-1-2-16-17(8-14)23-18(20-16)9-15-12-4-10-3-11(6-12)7-13(15)5-10/h1-2,8-13H,3-7H2,(H2,19,21,22)/b15-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50098110
(CHEMBL11382 | Sulfamic acid 2-oxo-2H-chromen-7-yl ...)Show InChI InChI=1S/C9H7NO5S/c10-16(12,13)15-7-3-1-6-2-4-9(11)14-8(6)5-7/h1-5H,(H2,10,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human placental steroid sulfatase |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50525480
(CHEMBL4555404)Show SMILES NS(=O)(=O)Oc1ccc(CCNC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F)cc1 Show InChI InChI=1S/C19H11F21N2O4S/c20-10(21,9(43)42-6-5-7-1-3-8(4-2-7)46-47(41,44)45)11(22,23)12(24,25)13(26,27)14(28,29)15(30,31)16(32,33)17(34,35)18(36,37)19(38,39)40/h1-4H,5-6H2,(H,42,43)(H2,41,44,45) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human placental steroid sulfatase |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50097934

(CHEMBL84380 | Sulfamic acid 4-nonanoyl-phenyl este...)Show InChI InChI=1S/C15H23NO4S/c1-2-3-4-5-6-7-8-15(17)13-9-11-14(12-10-13)20-21(16,18)19/h9-12H,2-8H2,1H3,(H2,16,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) |
Eur J Med Chem 179: 257-271 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.052
BindingDB Entry DOI: 10.7270/Q2B27ZQ2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data