 Found 17 hits Enz. Inhib. hit(s) with all data for entry = 50010943
Found 17 hits Enz. Inhib. hit(s) with all data for entry = 50010943 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM20800
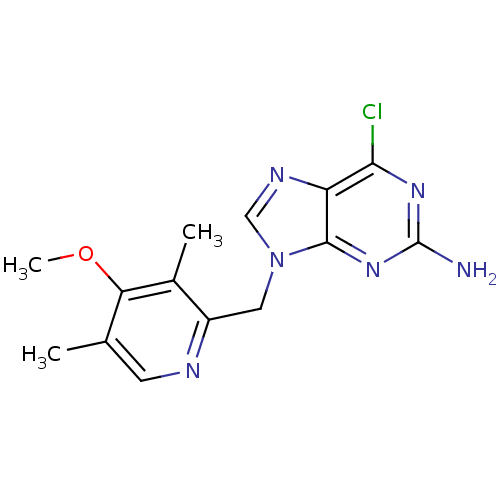
(2-amino-6-halopurine analogue, 20 | 6-chloro-9-[(4...)Show InChI InChI=1S/C14H15ClN6O/c1-7-4-17-9(8(2)11(7)22-3)5-21-6-18-10-12(15)19-14(16)20-13(10)21/h4,6H,5H2,1-3H3,(H2,16,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged full length human HSP90 (9 to 236 residues) expressed in Escherichia coli BL21(DE3) cells after 18 hrs by fluore... |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50543601

(CHEMBL2134202)Show SMILES CCc1cncnc1N1CCN(Cc2nc3cc(ccc3[nH]2)C(F)(F)F)CC1 Show InChI InChI=1S/C19H21F3N6/c1-2-13-10-23-12-24-18(13)28-7-5-27(6-8-28)11-17-25-15-4-3-14(19(20,21)22)9-16(15)26-17/h3-4,9-10,12H,2,5-8,11H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of S6K1 (unknown origin) |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM36609
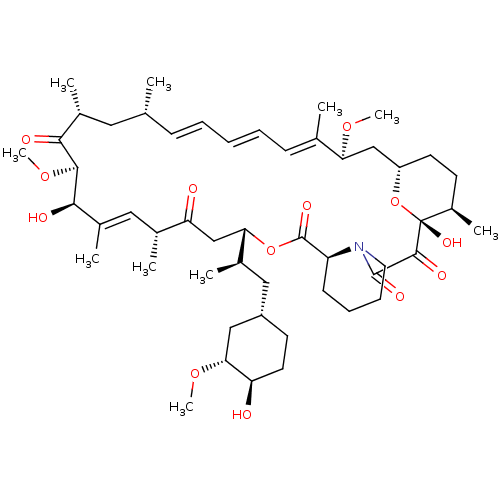
(Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in HEK293 cells |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50088378
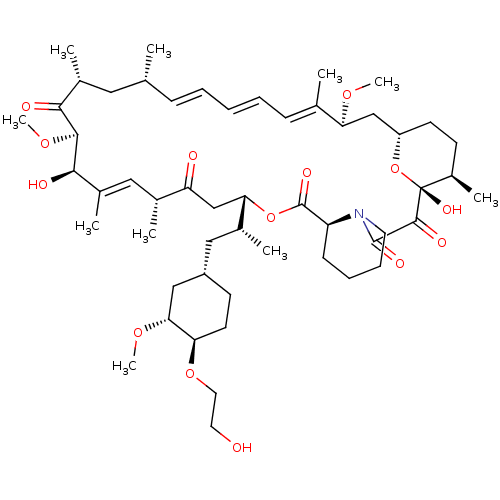
(Afinitor | Afinitor Disperz | CHEBI:68478 | Everol...)Show SMILES [H][C@@]12CC[C@@H](C)[C@@](O)(O1)C(=O)C(=O)N1CCCC[C@@]1([H])C(=O)O[C@@]([H])(CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C2)OC)[C@H](C)C[C@@H]1CC[C@@H](OCCO)[C@@H](C1)OC |r,c:32,51,t:47,49| Show InChI InChI=1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/b13-11+,16-12+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44+,45+,46-,48-,49+,53-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in HEK293 cells |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM66431
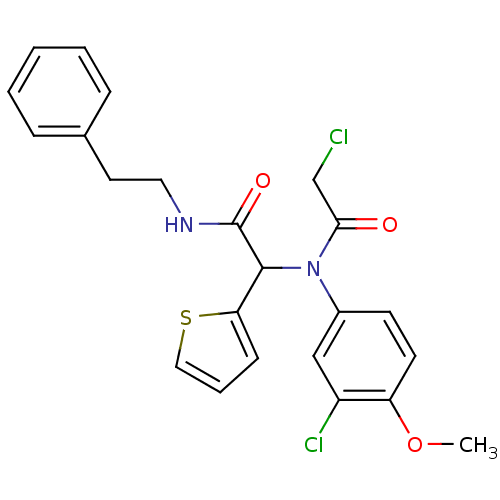
(2-(3-chloro-N-(2-chloro-1-oxoethyl)-4-methoxyanili...)Show SMILES COc1ccc(cc1Cl)N(C(C(=O)NCCc1ccccc1)c1cccs1)C(=O)CCl Show InChI InChI=1S/C23H22Cl2N2O3S/c1-30-19-10-9-17(14-18(19)25)27(21(28)15-24)22(20-8-5-13-31-20)23(29)26-12-11-16-6-3-2-4-7-16/h2-10,13-14,22H,11-12,15H2,1H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Vps34 (unknown origin) interaction with PIK3 assessed as reduction in autophagy by increasing LC3 level |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50087135

(CHEMBL3426621)Show SMILES C[C@H]1CNCCCN1S(=O)(=O)c1cccc2cncc(F)c12 |r| Show InChI InChI=1S/C15H18FN3O2S/c1-11-8-17-6-3-7-19(11)22(20,21)14-5-2-4-12-9-18-10-13(16)15(12)14/h2,4-5,9-11,17H,3,6-8H2,1H3/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50543600
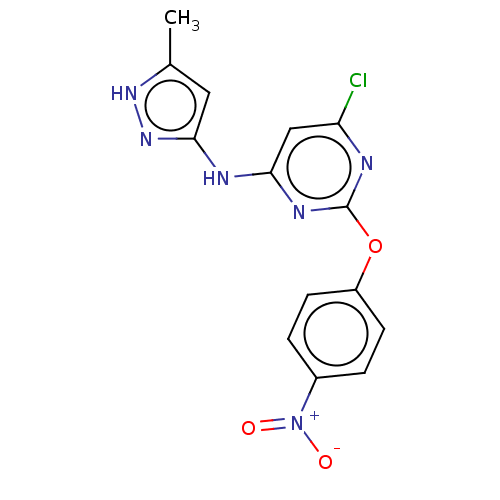
(CHEMBL4639853)Show SMILES Cc1cc(Nc2cc(Cl)nc(Oc3ccc(cc3)[N+]([O-])=O)n2)n[nH]1 Show InChI InChI=1S/C14H11ClN6O3/c1-8-6-13(20-19-8)17-12-7-11(15)16-14(18-12)24-10-4-2-9(3-5-10)21(22)23/h2-7H,1H3,(H2,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Vps34 in human MCF7-LC3 cells |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM66431

(2-(3-chloro-N-(2-chloro-1-oxoethyl)-4-methoxyanili...)Show SMILES COc1ccc(cc1Cl)N(C(C(=O)NCCc1ccccc1)c1cccs1)C(=O)CCl Show InChI InChI=1S/C23H22Cl2N2O3S/c1-30-19-10-9-17(14-18(19)25)27(21(28)15-24)22(20-8-5-13-31-20)23(29)26-12-11-16-6-3-2-4-7-16/h2-10,13-14,22H,11-12,15H2,1H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Vps34 (unknown origin) |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50543600

(CHEMBL4639853)Show SMILES Cc1cc(Nc2cc(Cl)nc(Oc3ccc(cc3)[N+]([O-])=O)n2)n[nH]1 Show InChI InChI=1S/C14H11ClN6O3/c1-8-6-13(20-19-8)17-12-7-11(15)16-14(18-12)24-10-4-2-9(3-5-10)21(22)23/h2-7H,1H3,(H2,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Vps34 in human MCF7-LC3 cells assessed as rapamycin-induced autophagy |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50087135

(CHEMBL3426621)Show SMILES C[C@H]1CNCCCN1S(=O)(=O)c1cccc2cncc(F)c12 |r| Show InChI InChI=1S/C15H18FN3O2S/c1-11-8-17-6-3-7-19(11)22(20,21)14-5-2-4-12-9-18-10-13(16)15(12)14/h2,4-5,9-11,17H,3,6-8H2,1H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50543600

(CHEMBL4639853)Show SMILES Cc1cc(Nc2cc(Cl)nc(Oc3ccc(cc3)[N+]([O-])=O)n2)n[nH]1 Show InChI InChI=1S/C14H11ClN6O3/c1-8-6-13(20-19-8)17-12-7-11(15)16-14(18-12)24-10-4-2-9(3-5-10)21(22)23/h2-7H,1H3,(H2,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Vps34 in human MCF7-LC3 cells assessed as starvation-induced autophagy |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50543601

(CHEMBL2134202)Show SMILES CCc1cncnc1N1CCN(Cc2nc3cc(ccc3[nH]2)C(F)(F)F)CC1 Show InChI InChI=1S/C19H21F3N6/c1-2-13-10-23-12-24-18(13)28-7-5-27(6-8-28)11-17-25-15-4-3-14(19(20,21)22)9-16(15)26-17/h3-4,9-10,12H,2,5-8,11H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of S6K1 (unknown origin) |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-glucuronosyltransferase 1A1
(Homo sapiens (Human)) | BDBM50068270
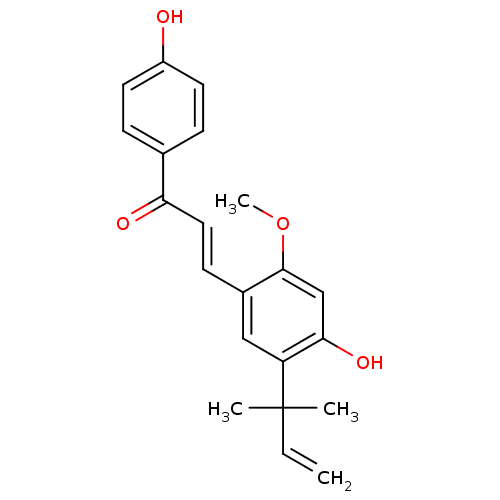
((E)-3-(4-hydroxy-2-methoxy-5-(2-methylbut-3-en-2-y...)Show SMILES COc1cc(O)c(cc1\C=C\C(=O)c1ccc(O)cc1)C(C)(C)C=C Show InChI InChI=1S/C21H22O4/c1-5-21(2,3)17-12-15(20(25-4)13-19(17)24)8-11-18(23)14-6-9-16(22)10-7-14/h5-13,22,24H,1H2,2-4H3/b11-8+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Induction of UGT1A1 (unknown origin) assessed as increase in intracellular acidic autophagy vesicles formation |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |
Kelch-like ECH-associated protein 1
(Homo sapiens (Human)) | BDBM50205338
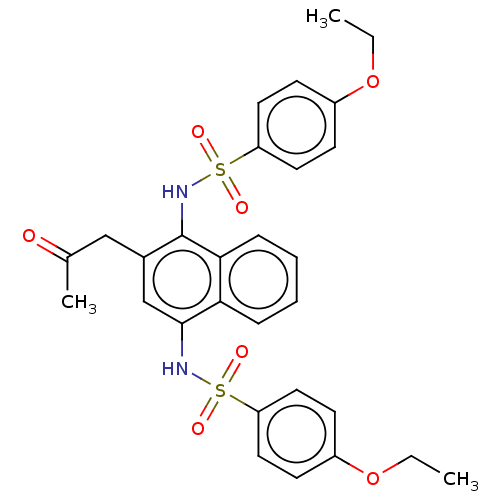
(CHEMBL3948237)Show SMILES CCOc1ccc(cc1)S(=O)(=O)Nc1cc(CC(C)=O)c(NS(=O)(=O)c2ccc(OCC)cc2)c2ccccc12 Show InChI InChI=1S/C29H30N2O7S2/c1-4-37-22-10-14-24(15-11-22)39(33,34)30-28-19-21(18-20(3)32)29(27-9-7-6-8-26(27)28)31-40(35,36)25-16-12-23(13-17-25)38-5-2/h6-17,19,30-31H,4-5,18H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of GST/His-tagged human KEAP1 (321 to 609 residues) interaction with phosphorylated p62 expressed in Escherichia coli cells by fluorescenc... |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase-alpha (unknown origin) |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50017716
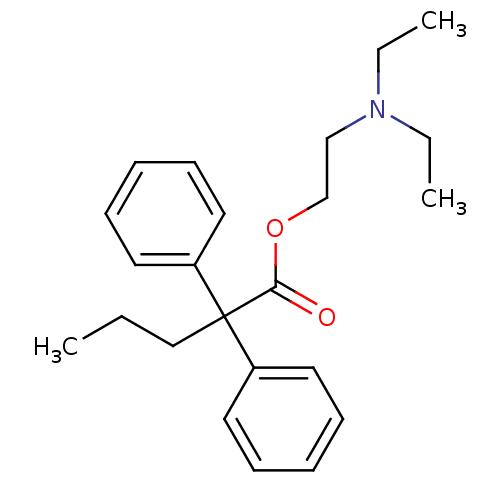
(2,2-Diphenyl-pentanoic acid 2-diethylamino-ethyl e...)Show InChI InChI=1S/C23H31NO2/c1-4-17-23(20-13-9-7-10-14-20,21-15-11-8-12-16-21)22(25)26-19-18-24(5-2)6-3/h7-16H,4-6,17-19H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 (unknown origin) |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data