 Found 38 hits Enz. Inhib. hit(s) with all data for entry = 50013219
Found 38 hits Enz. Inhib. hit(s) with all data for entry = 50013219 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50065646

(CHEMBL3087181)Show SMILES O=C(N1CCC(CC1)C(c1ccc2OCOc2c1)c1ccc2OCOc2c1)n1cncn1 Show InChI InChI=1S/C23H22N4O5/c28-23(27-12-24-11-25-27)26-7-5-15(6-8-26)22(16-1-3-18-20(9-16)31-13-29-18)17-2-4-19-21(10-17)32-14-30-19/h1-4,9-12,15,22H,5-8,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50242634

(5-((biphenyl-4-yl)methyl)-N,N-dimethyl-2H-tetrazol...)Show InChI InChI=1S/C17H17N5O/c1-21(2)17(23)22-19-16(18-20-22)12-13-8-10-15(11-9-13)14-6-4-3-5-7-14/h3-11H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAGL (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM179929

(US9133148, 1a)Show SMILES OC(C1CCN(CC1)C(=O)OC(C(F)(F)F)C(F)(F)F)(c1ccc2OCOc2c1)c1ccc2OCOc2c1 Show InChI InChI=1S/C24H21F6NO7/c25-23(26,27)20(24(28,29)30)38-21(32)31-7-5-13(6-8-31)22(33,14-1-3-16-18(9-14)36-11-34-16)15-2-4-17-19(10-15)37-12-35-17/h1-4,9-10,13,20,33H,5-8,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL transfected in human HEK293T cells assessed as reduction in ABPP binding by competitive binding assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM60622
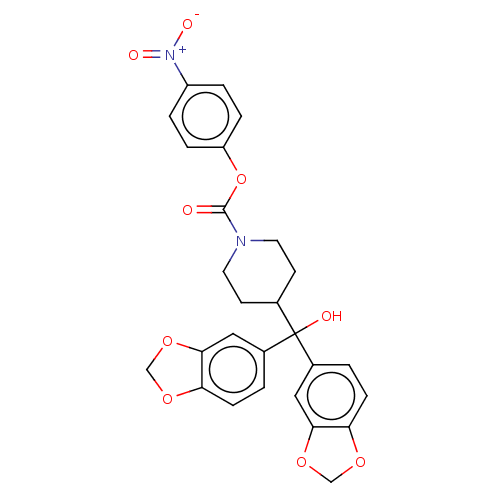
(BDBM50300355 | US11753371, Compound JZL-184 | US91...)Show SMILES OC(C1CCN(CC1)C(=O)Oc1ccc(cc1)[N+]([O-])=O)(c1ccc2OCOc2c1)c1ccc2OCOc2c1 Show InChI InChI=1S/C27H24N2O9/c30-26(38-21-5-3-20(4-6-21)29(32)33)28-11-9-17(10-12-28)27(31,18-1-7-22-24(13-18)36-15-34-22)19-2-8-23-25(14-19)37-16-35-23/h1-8,13-14,17,31H,9-12,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAGL (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563791
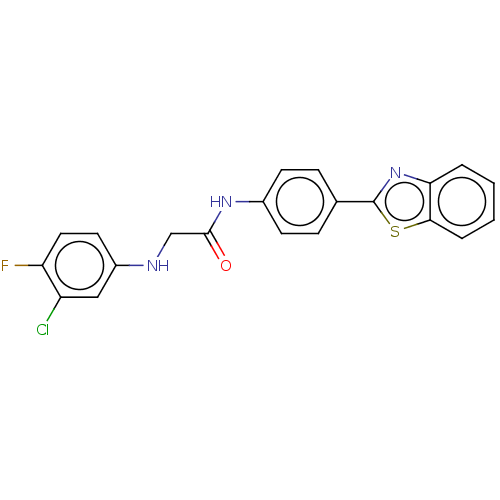
(CHEMBL4778803)Show SMILES Fc1ccc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)cc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563792
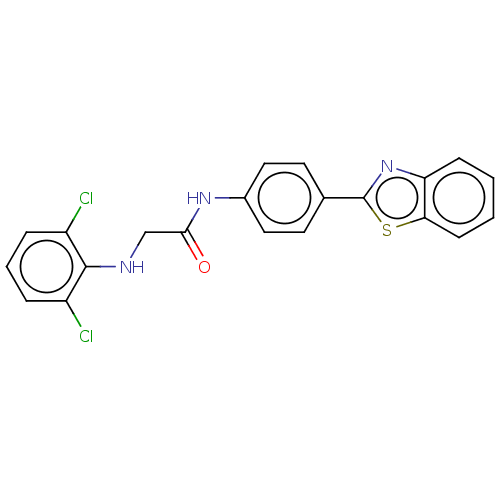
(CHEMBL4788586)Show SMILES Clc1cccc(Cl)c1NCC(=O)Nc1ccc(cc1)-c1nc2ccccc2s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563794
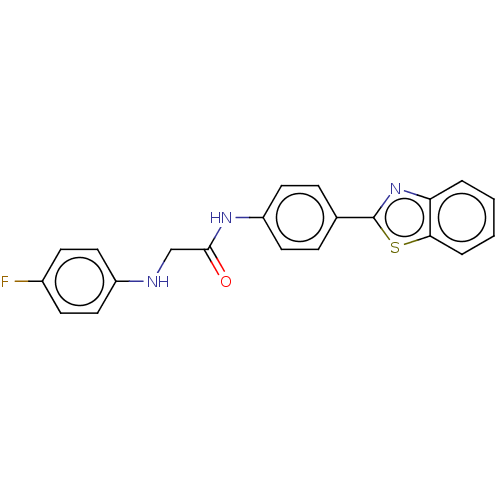
(CHEMBL4793668)Show SMILES Fc1ccc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563793

(CHEMBL4793997)Show SMILES Clc1ccc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563790

(CHEMBL4780294)Show SMILES Brc1ccc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563801
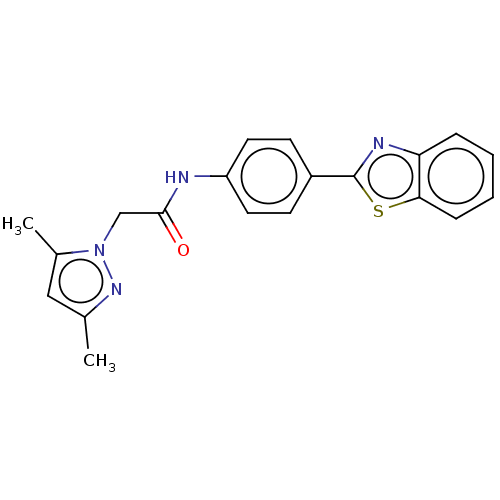
(CHEMBL4779678)Show SMILES Cc1cc(C)n(CC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563802
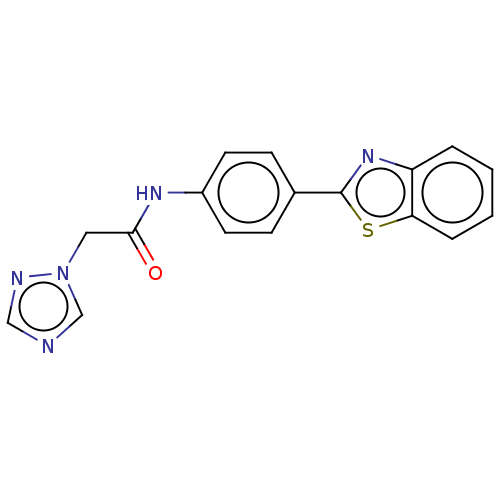
(CHEMBL4793265) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50414921

(CHEMBL570812)Show SMILES COc1nn(-c2ccc(NC(=O)OCc3ccccc3)c(C)c2)c(=O)o1 Show InChI InChI=1S/C18H17N3O5/c1-12-10-14(21-18(23)26-17(20-21)24-2)8-9-15(12)19-16(22)25-11-13-6-4-3-5-7-13/h3-10H,11H2,1-2H3,(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAGL (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563803
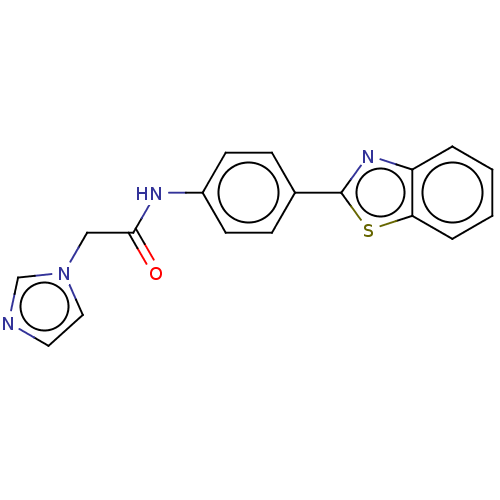
(CHEMBL4797565) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563795
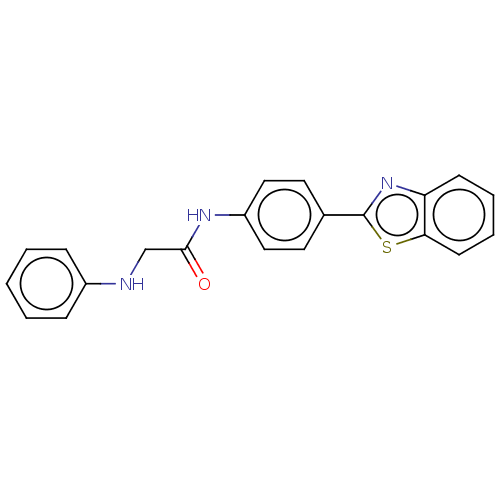
(CHEMBL4786239) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563785

(CHEMBL4792393)Show SMILES NS(=O)(=O)c1ccc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50432829

(CHEMBL2171700)Show InChI InChI=1S/C15H12N2O4/c1-19-14-16-17(15(18)21-14)11-7-9-13(10-8-11)20-12-5-3-2-4-6-12/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAGL (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563809

(CHEMBL4794817)Show SMILES O=C(Cn1cnc2ccccc12)Nc1ccc(cc1)-c1nc2ccccc2s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563797
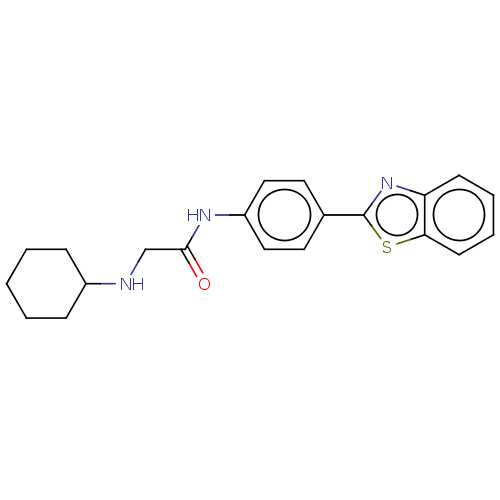
(CHEMBL4777523) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563808
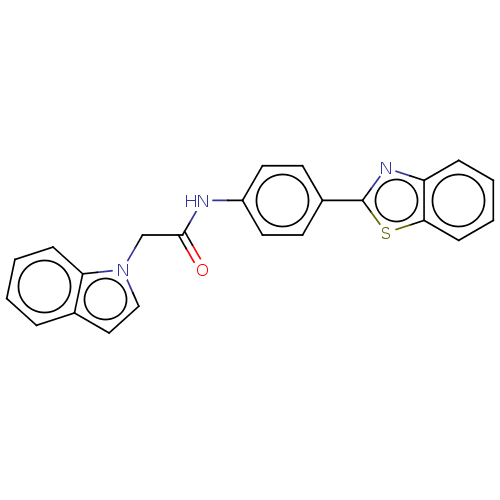
(CHEMBL4793077)Show SMILES O=C(Cn1ccc2ccccc12)Nc1ccc(cc1)-c1nc2ccccc2s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563796

(CHEMBL4780477) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563800

(CHEMBL4776276) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50414921

(CHEMBL570812)Show SMILES COc1nn(-c2ccc(NC(=O)OCc3ccccc3)c(C)c2)c(=O)o1 Show InChI InChI=1S/C18H17N3O5/c1-12-10-14(21-18(23)26-17(20-21)24-2)8-9-15(12)19-16(22)25-11-13-6-4-3-5-7-13/h3-10H,11H2,1-2H3,(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 424 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563799
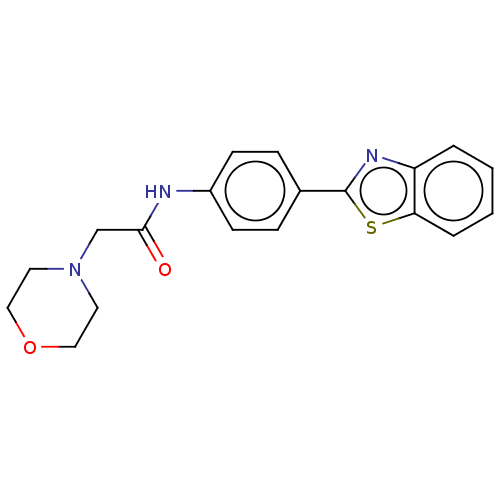
(CHEMBL4785038) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563798

(CHEMBL4800406) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 815 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563788
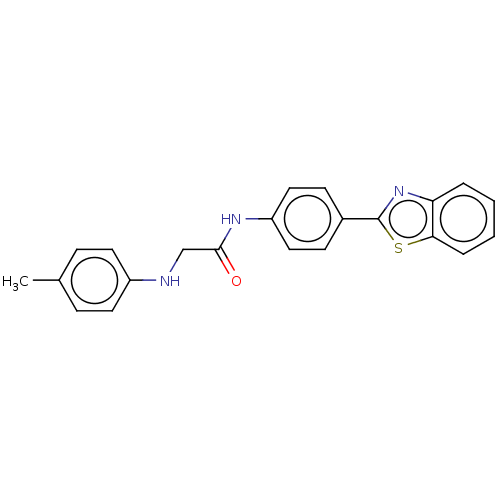
(CHEMBL4798413)Show SMILES Cc1ccc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563787

(CHEMBL4788417)Show SMILES COc1ccc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563786

(CHEMBL4783652)Show SMILES Oc1ccc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563782
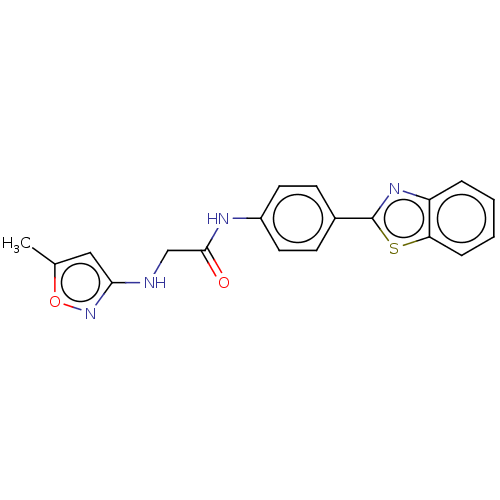
(CHEMBL4786879) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50128581

(Biphenyl-3-yl-carbamic acid cyclohexyl ester | CHE...)Show InChI InChI=1S/C19H21NO2/c21-19(22-18-12-5-2-6-13-18)20-17-11-7-10-16(14-17)15-8-3-1-4-9-15/h1,3-4,7-11,14,18H,2,5-6,12-13H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAGL (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50274981
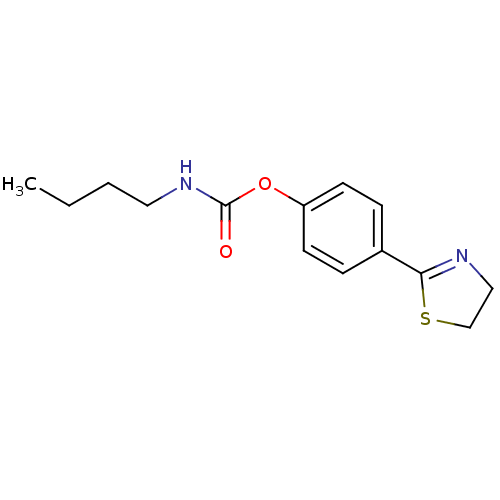
(4-(4,5-dihydrothiazol-2-yl)phenyl butylcarbamate |...)Show InChI InChI=1S/C14H18N2O2S/c1-2-3-8-16-14(17)18-12-6-4-11(5-7-12)13-15-9-10-19-13/h4-7H,2-3,8-10H2,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAGL (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563781
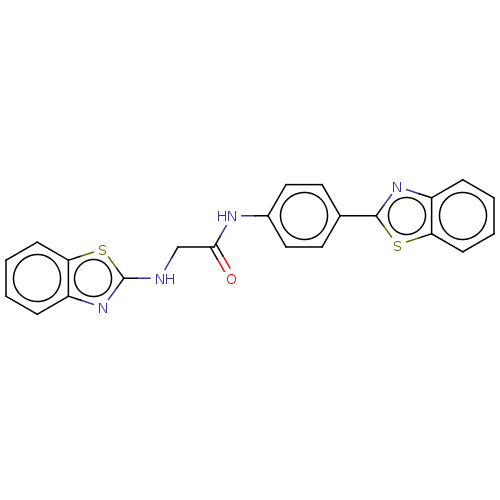
(CHEMBL4800077)Show SMILES O=C(CNc1nc2ccccc2s1)Nc1ccc(cc1)-c1nc2ccccc2s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563784
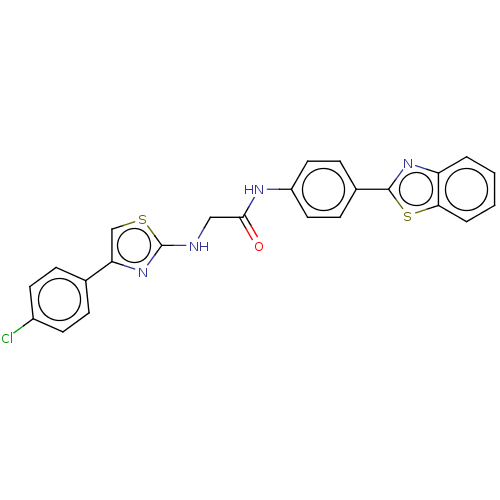
(CHEMBL4796478)Show SMILES Clc1ccc(cc1)-c1csc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563783
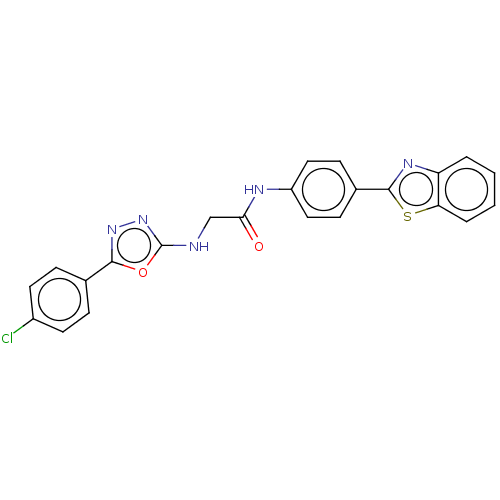
(CHEMBL4777889)Show SMILES Clc1ccc(cc1)-c1nnc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563807
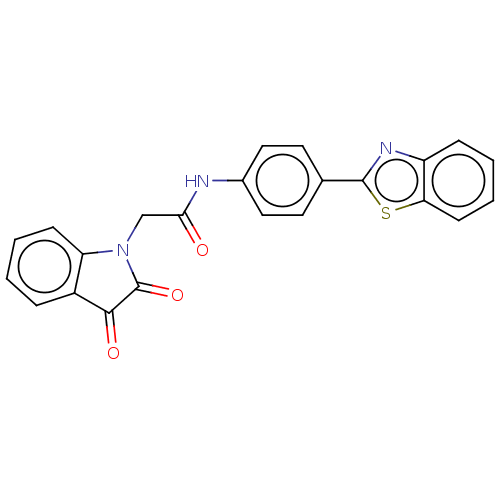
(CHEMBL4777402)Show SMILES O=C(CN1C(=O)C(=O)c2ccccc12)Nc1ccc(cc1)-c1nc2ccccc2s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563806
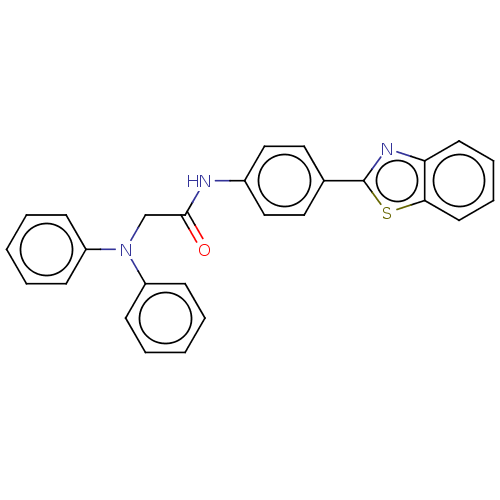
(CHEMBL4783153)Show SMILES O=C(CN(c1ccccc1)c1ccccc1)Nc1ccc(cc1)-c1nc2ccccc2s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563804
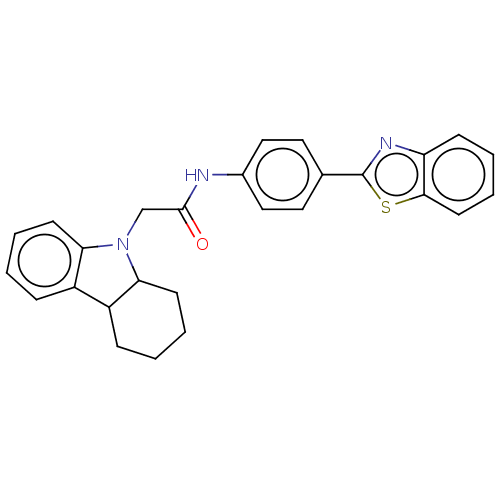
(CHEMBL4800531)Show SMILES O=C(CN1C2CCCCC2c2ccccc12)Nc1ccc(cc1)-c1nc2ccccc2s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563789
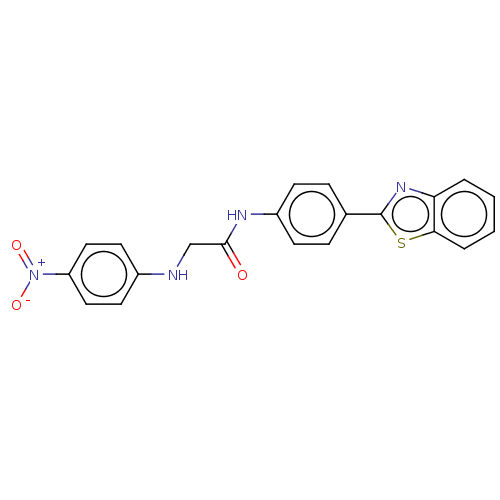
(CHEMBL4799435)Show SMILES [O-][N+](=O)c1ccc(NCC(=O)Nc2ccc(cc2)-c2nc3ccccc3s2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50563805
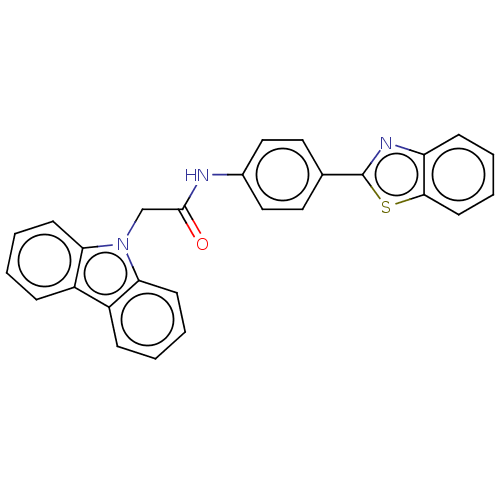
(CHEMBL4784727)Show SMILES O=C(Cn1c2ccccc2c2ccccc12)Nc1ccc(cc1)-c1nc2ccccc2s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAGL using 4-Nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.ejmech.2016.05.038
BindingDB Entry DOI: 10.7270/Q28056BZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data