 Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50015022
Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50015022 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM532292
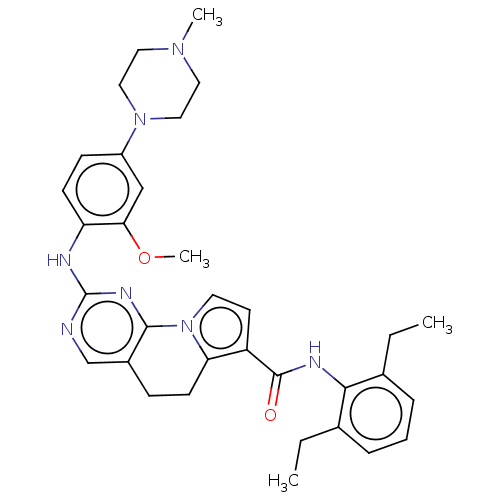
(JGS79C | N-(2,6-diethylphenyl)-2-[2-methoxy-4-(4-m...)Show SMILES CCc1cccc(CC)c1NC(=O)c1ccn-2c1CCc1cnc(Nc3ccc(cc3OC)N3CCN(C)CC3)nc-21 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length TTK (unknown origin) using MBP-derived peptide as substrate preincubated for 1 hr in dark followed by substrate addition an... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582067
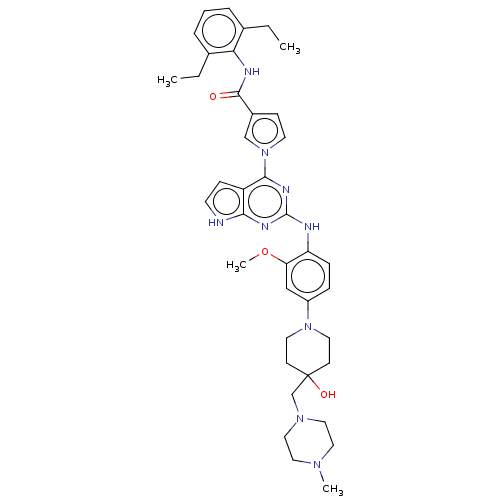
(CHEMBL5093015)Show SMILES CCc1cccc(CC)c1NC(=O)c1ccn(c1)-c1nc(Nc2ccc(cc2OC)N2CCC(O)(CN3CCN(C)CC3)CC2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582063
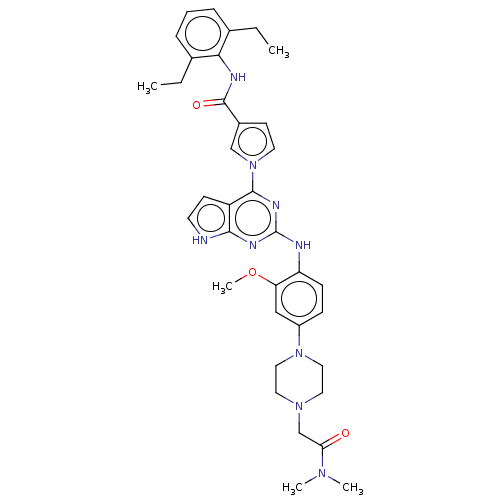
(CHEMBL5092826)Show SMILES CCc1cccc(CC)c1NC(=O)c1ccn(c1)-c1nc(Nc2ccc(cc2OC)N2CCN(CC(=O)N(C)C)CC2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582069
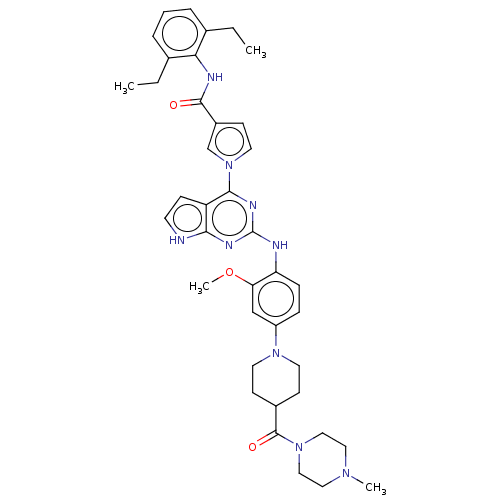
(CHEMBL5073101)Show SMILES CCc1cccc(CC)c1NC(=O)c1ccn(c1)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CCN(C)CC2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582068
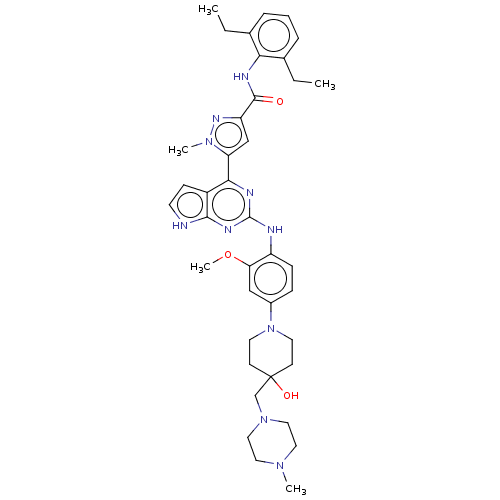
(CHEMBL5077668)Show SMILES CCc1cccc(CC)c1NC(=O)c1cc(-c2nc(Nc3ccc(cc3OC)N3CCC(O)(CN4CCN(C)CC4)CC3)nc3[nH]ccc23)n(C)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582065

(CHEMBL5075313)Show SMILES CCc1cccc(CC)c1NC(=O)c1ccn(c1)-c1nc(Nc2ccc(cc2OC)N2CCC(O)(CC2)C(=O)N2CCN(C)CC2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582061
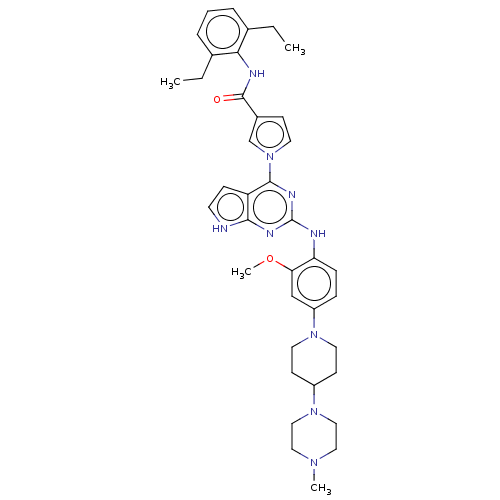
(CHEMBL5081270)Show SMILES CCc1cccc(CC)c1NC(=O)c1ccn(c1)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)N2CCN(C)CC2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582074

(CHEMBL5081668)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(O)(CN3CCN(C)CC3)CC2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582077
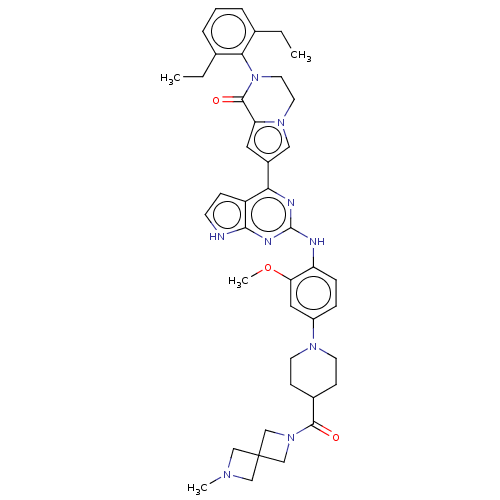
(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582066
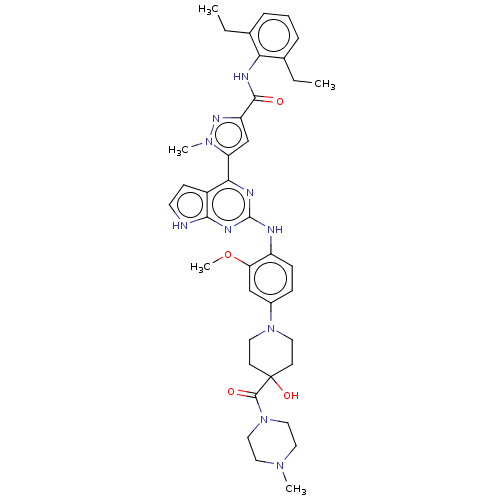
(CHEMBL5084062)Show SMILES CCc1cccc(CC)c1NC(=O)c1cc(-c2nc(Nc3ccc(cc3OC)N3CCC(O)(CC3)C(=O)N3CCN(C)CC3)nc3[nH]ccc23)n(C)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582064
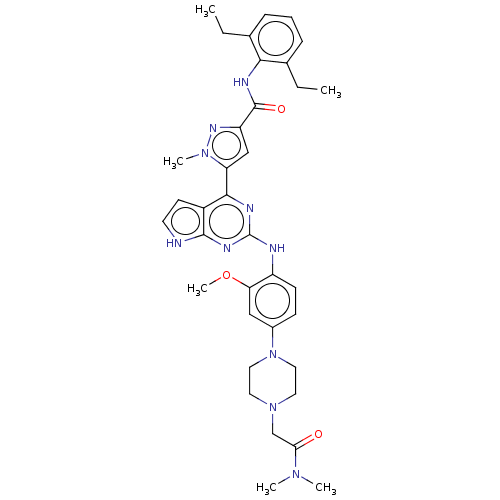
(CHEMBL5091689)Show SMILES CCc1cccc(CC)c1NC(=O)c1cc(-c2nc(Nc3ccc(cc3OC)N3CCN(CC(=O)N(C)C)CC3)nc3[nH]ccc23)n(C)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582070

(CHEMBL5085278)Show SMILES CCc1cccc(CC)c1NC(=O)c1cc(-c2nc(Nc3ccc(cc3OC)N3CCC(CC3)C(=O)N3CCN(C)CC3)nc3[nH]ccc23)n(C)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582071

(CHEMBL5086022)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)N2CCN(C)CC2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582076
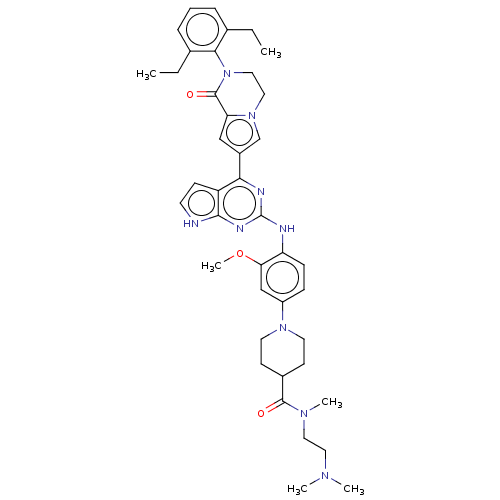
(CHEMBL5079606)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N(C)CCN(C)C)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582075
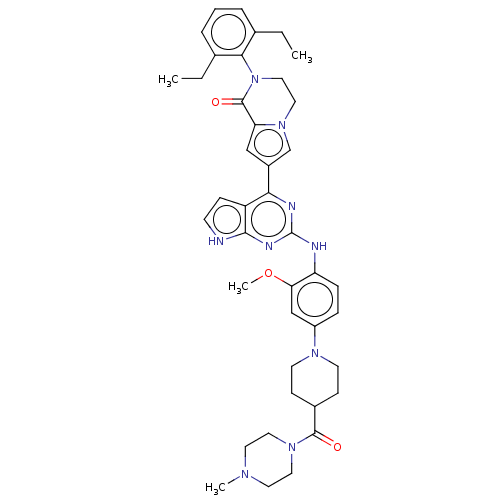
(CHEMBL5085182)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CCN(C)CC2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582062
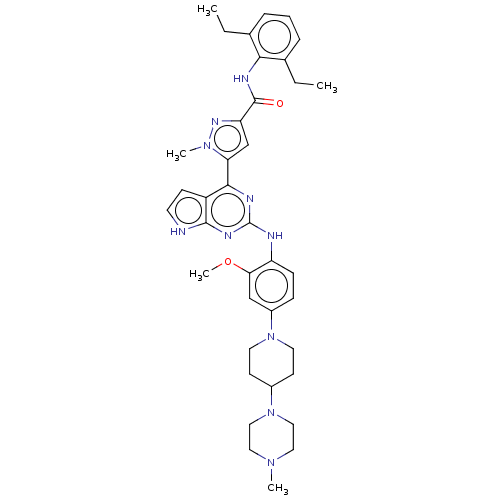
(CHEMBL5086435)Show SMILES CCc1cccc(CC)c1NC(=O)c1cc(-c2nc(Nc3ccc(cc3OC)N3CCC(CC3)N3CCN(C)CC3)nc3[nH]ccc23)n(C)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582073
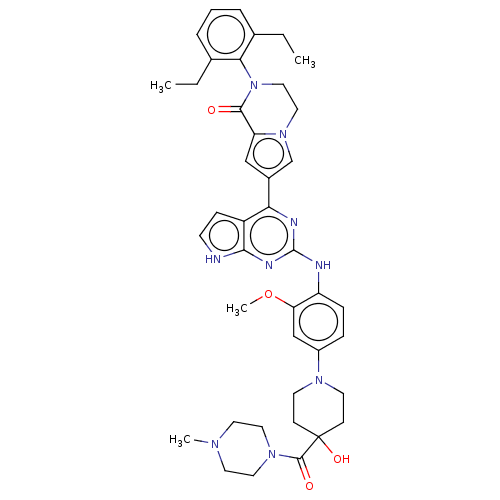
(CHEMBL5077627)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(O)(CC2)C(=O)N2CCN(C)CC2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582072

(CHEMBL5087340)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCN(CC(=O)N(C)C)CC2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer 236 from TTK (unknown origin) by LanthaScreen Eu kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Testis-specific serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50582077

(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TSSK1 (unknown origin) by Z-lyte assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50582077

(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TTK autophosphorylation at T686 residue in human CAL-51 cells measured after 1 hr by Western blot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50582077

(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 192 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK1 (unknown origin) by Z-lyte assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582077

(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LRRK2 G2019S mutant (unknown origin) by ADAPTA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM317462
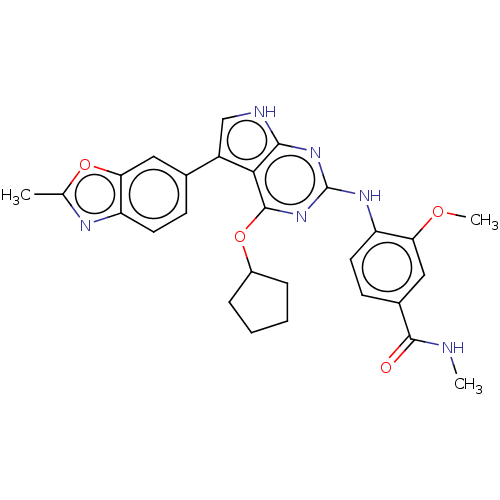
(4-(4-(cyclopentyloxy)-5-(2- methylbenzo[d]oxazol-6...)Show SMILES CNC(=O)c1ccc(Nc2nc(OC3CCCC3)c3c(c[nH]c3n2)-c2ccc3nc(C)oc3c2)c(OC)c1 Show InChI InChI=1S/C28H28N6O4/c1-15-31-21-10-8-16(12-23(21)37-15)19-14-30-25-24(19)27(38-18-6-4-5-7-18)34-28(33-25)32-20-11-9-17(26(35)29-2)13-22(20)36-3/h8-14,18H,4-7H2,1-3H3,(H,29,35)(H2,30,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582077

(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 342 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LRRK2 (unknown origin) by ADAPTA assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50582077

(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 545 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CHK2 (unknown origin) by Z-lyte assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50582077

(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 602 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of JNK2 (unknown origin) by Z-lyte assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Testis-specific serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50582077

(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 944 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TSSK2 (unknown origin) by Z-lyte assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582067

(CHEMBL5093015)Show SMILES CCc1cccc(CC)c1NC(=O)c1ccn(c1)-c1nc(Nc2ccc(cc2OC)N2CCC(O)(CN3CCN(C)CC3)CC2)nc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50582077

(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SRC N1 (unknown origin) by Z-lyte assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50582077

(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MAPKAPK3 (unknown origin) by Z-lyte assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582065

(CHEMBL5075313)Show SMILES CCc1cccc(CC)c1NC(=O)c1ccn(c1)-c1nc(Nc2ccc(cc2OC)N2CCC(O)(CC2)C(=O)N2CCN(C)CC2)nc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582061

(CHEMBL5081270)Show SMILES CCc1cccc(CC)c1NC(=O)c1ccn(c1)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)N2CCN(C)CC2)nc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582069

(CHEMBL5073101)Show SMILES CCc1cccc(CC)c1NC(=O)c1ccn(c1)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CCN(C)CC2)nc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582076

(CHEMBL5079606)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N(C)CCN(C)C)nc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582077

(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582075

(CHEMBL5085182)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CCN(C)CC2)nc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582074

(CHEMBL5081668)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(O)(CN3CCN(C)CC3)CC2)nc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582073

(CHEMBL5077627)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(O)(CC2)C(=O)N2CCN(C)CC2)nc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582071

(CHEMBL5086022)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)N2CCN(C)CC2)nc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582070

(CHEMBL5085278)Show SMILES CCc1cccc(CC)c1NC(=O)c1cc(-c2nc(Nc3ccc(cc3OC)N3CCC(CC3)C(=O)N3CCN(C)CC3)nc3[nH]ccc23)n(C)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582068

(CHEMBL5077668)Show SMILES CCc1cccc(CC)c1NC(=O)c1cc(-c2nc(Nc3ccc(cc3OC)N3CCC(O)(CN4CCN(C)CC4)CC3)nc3[nH]ccc23)n(C)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582062

(CHEMBL5086435)Show SMILES CCc1cccc(CC)c1NC(=O)c1cc(-c2nc(Nc3ccc(cc3OC)N3CCC(CC3)N3CCN(C)CC3)nc3[nH]ccc23)n(C)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50512317
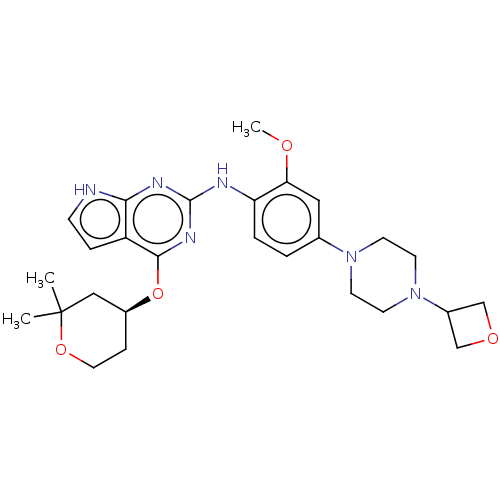
(CHEMBL4569626)Show SMILES COc1cc(ccc1Nc1nc(O[C@H]2CCOC(C)(C)C2)c2cc[nH]c2n1)N1CCN(CC1)C1COC1 |r| Show InChI InChI=1S/C27H36N6O4/c1-27(2)15-20(7-13-36-27)37-25-21-6-8-28-24(21)30-26(31-25)29-22-5-4-18(14-23(22)34-3)32-9-11-33(12-10-32)19-16-35-17-19/h4-6,8,14,19-20H,7,9-13,15-17H2,1-3H3,(H2,28,29,30,31)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50582066

(CHEMBL5084062)Show SMILES CCc1cccc(CC)c1NC(=O)c1cc(-c2nc(Nc3ccc(cc3OC)N3CCC(O)(CC3)C(=O)N3CCN(C)CC3)nc3[nH]ccc23)n(C)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using ketoconazole as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50582077

(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of hERG |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50582077

(CHEMBL5085753)Show SMILES CCc1cccc(CC)c1N1CCn2cc(cc2C1=O)-c1nc(Nc2ccc(cc2OC)N2CCC(CC2)C(=O)N2CC3(CN(C)C3)C2)nc2[nH]ccc12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Time dependent inhibition of CYP2C8 in human liver microsomes |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00635
BindingDB Entry DOI: 10.7270/Q25T3QC6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data