

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584634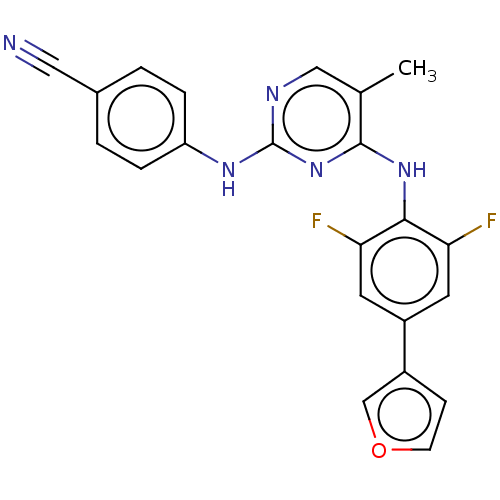 (CHEMBL5078684) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50103642 (4-(6-amino-5-bromo-2-(4-cyanophenylamino)pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584633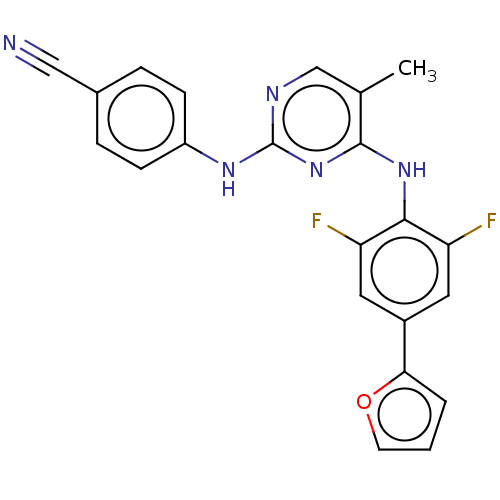 (CHEMBL5076567) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584630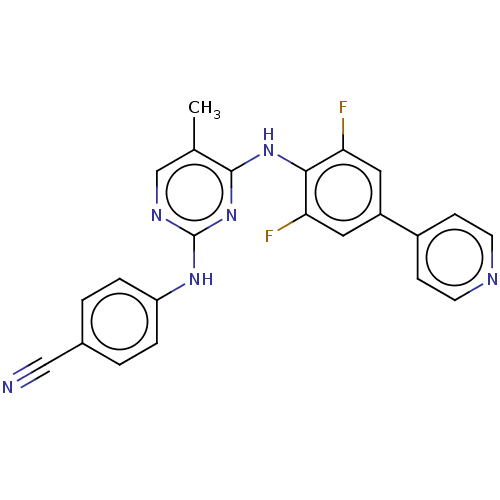 (CHEMBL5086160) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584635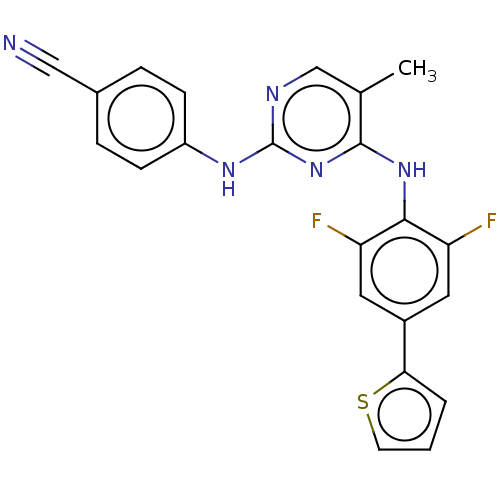 (CHEMBL5094298) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584636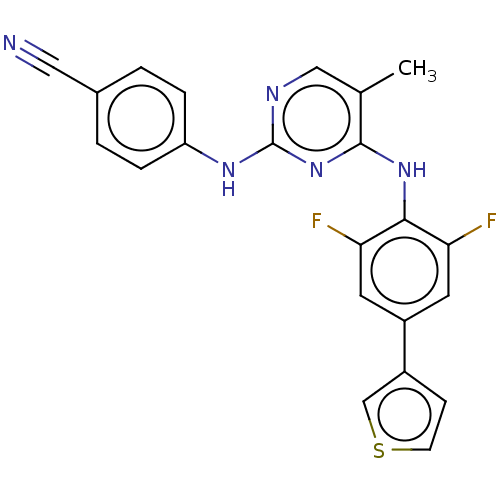 (CHEMBL5077589) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM222178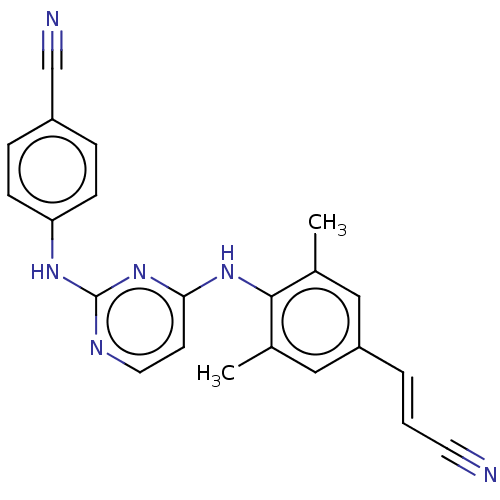 (Rilpivirine) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584641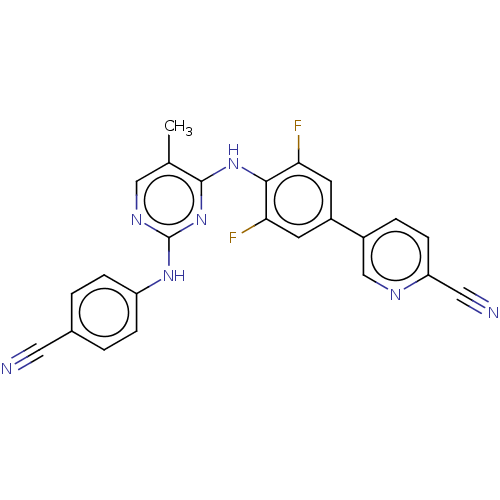 (CHEMBL5092151) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584643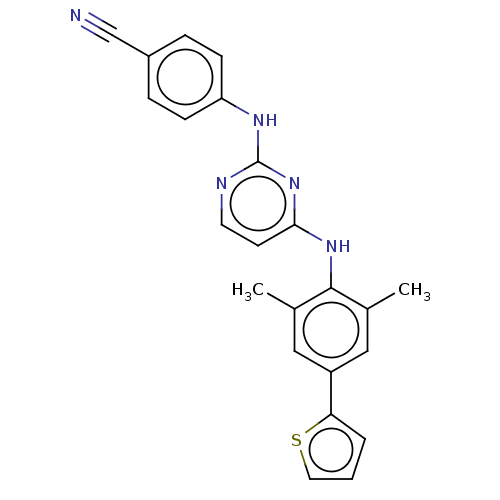 (CHEMBL5088915) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584645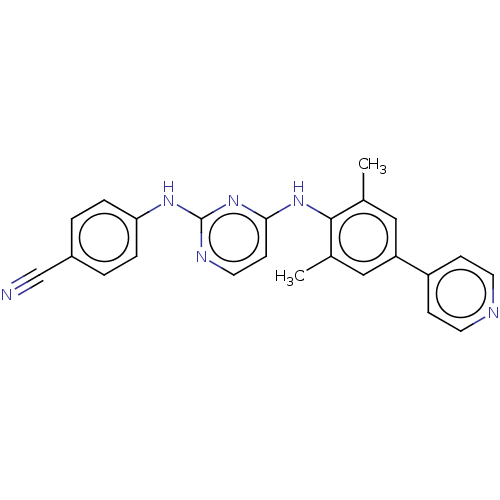 (CHEMBL5081783) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584653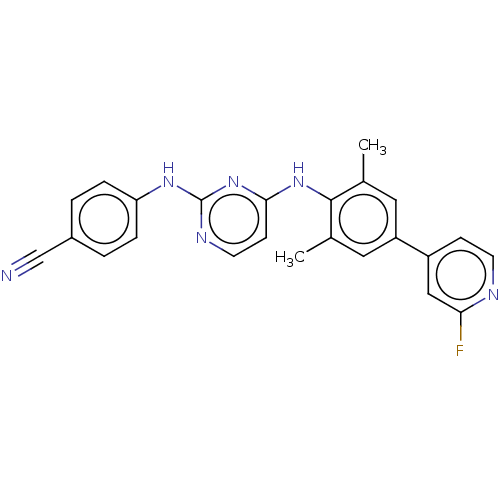 (CHEMBL5082832) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584652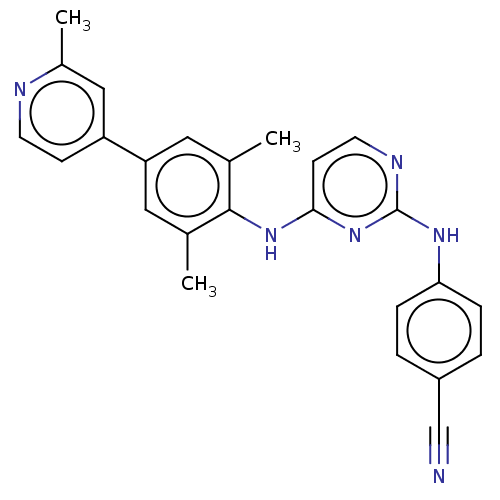 (CHEMBL5080518) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584637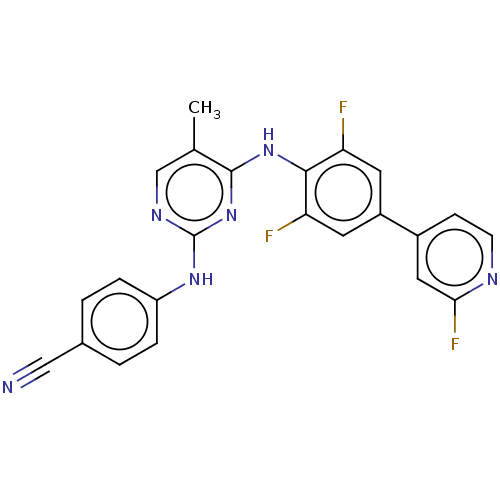 (CHEMBL5087871) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584658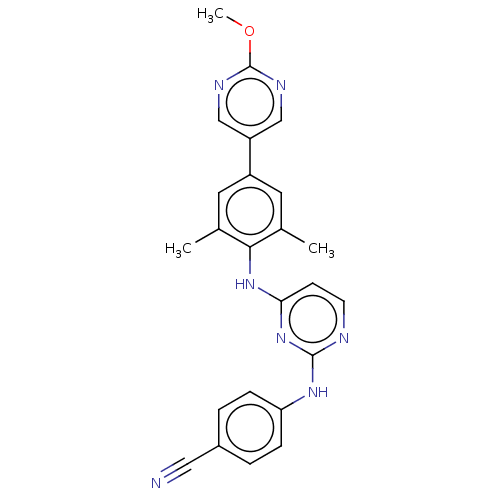 (CHEMBL5088410) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584642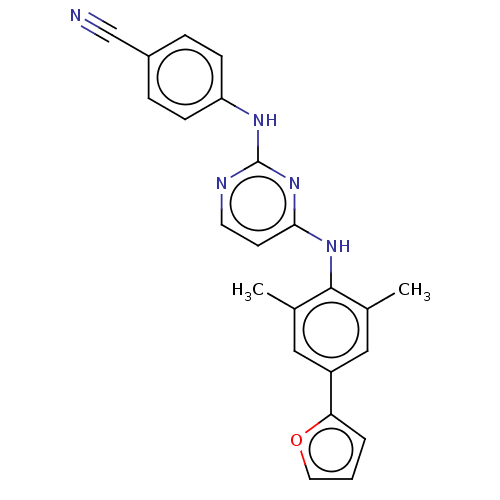 (CHEMBL5077783) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584659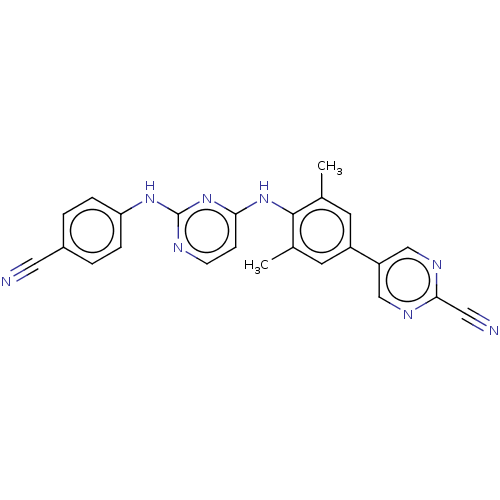 (CHEMBL5092491) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584647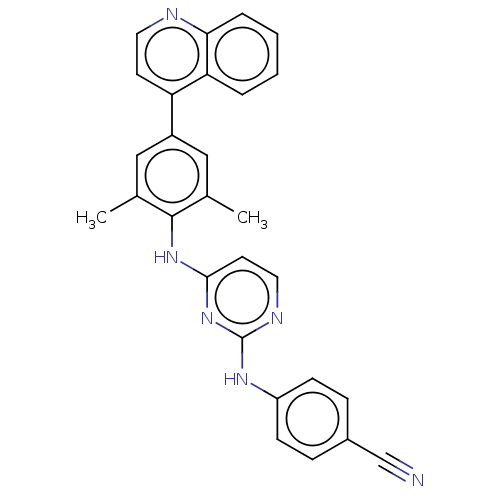 (CHEMBL5075761) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584651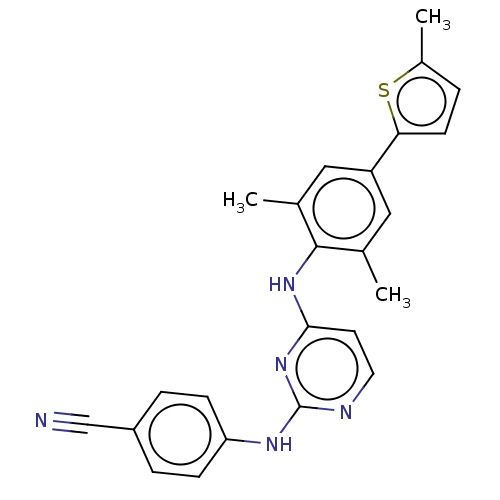 (CHEMBL5085325) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584656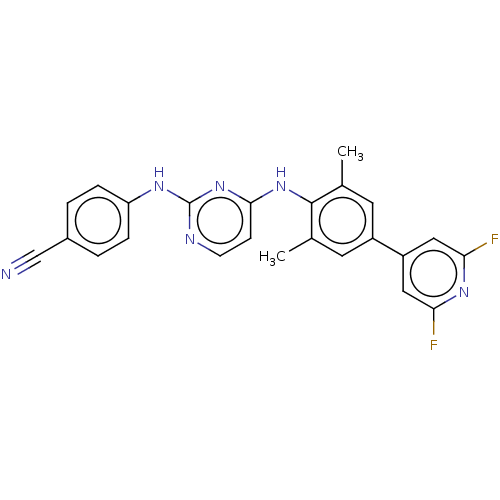 (CHEMBL5082632) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584657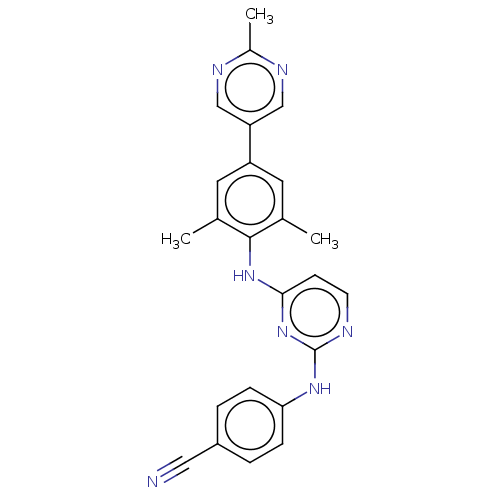 (CHEMBL5074781) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584649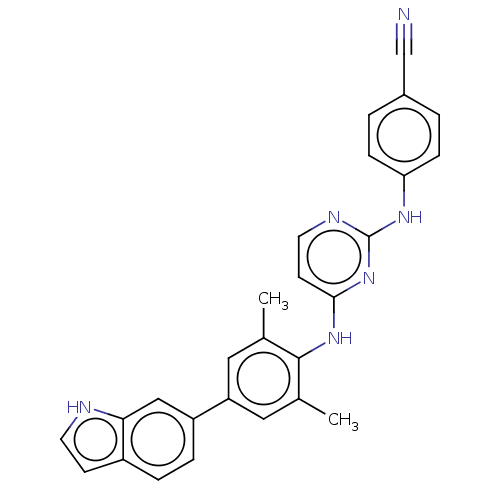 (CHEMBL5079307) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584638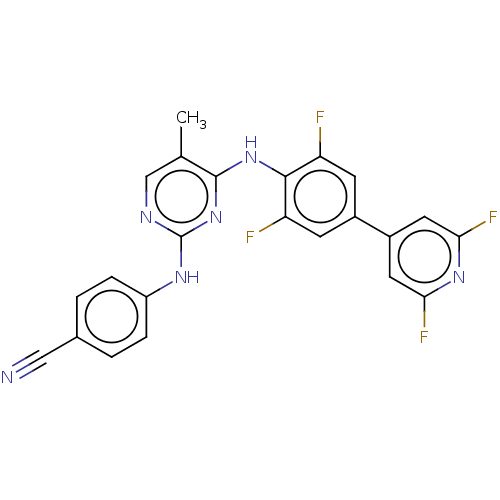 (CHEMBL5079060) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584650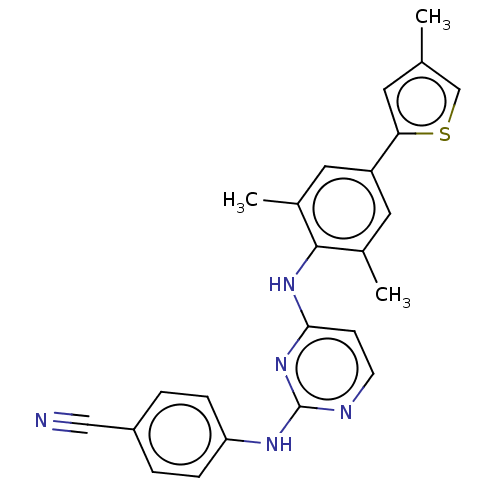 (CHEMBL5084031) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584648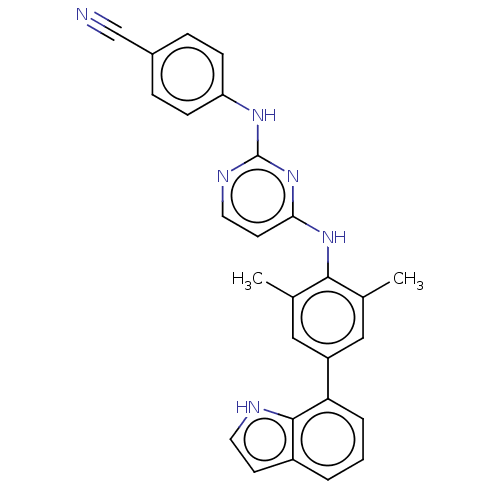 (CHEMBL5075154) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584646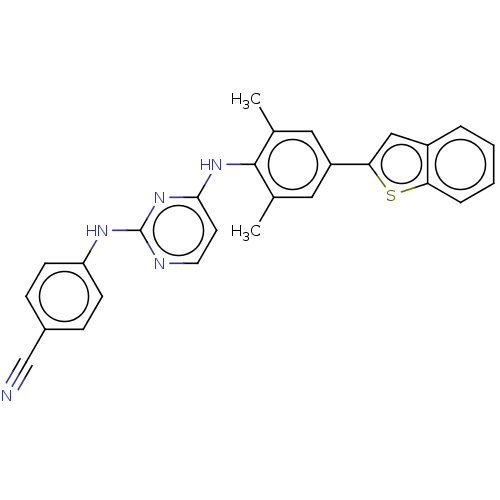 (CHEMBL5093657) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584660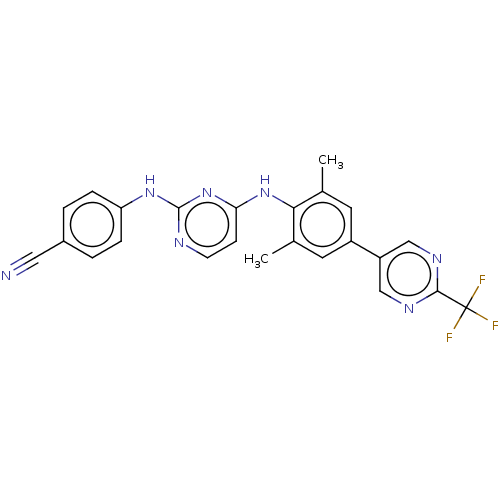 (CHEMBL5093150) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584655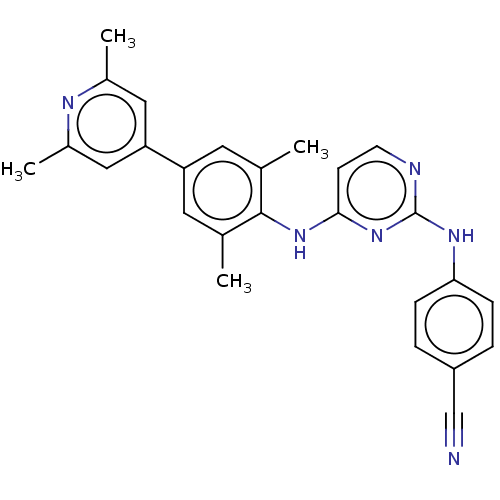 (CHEMBL5092468) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584654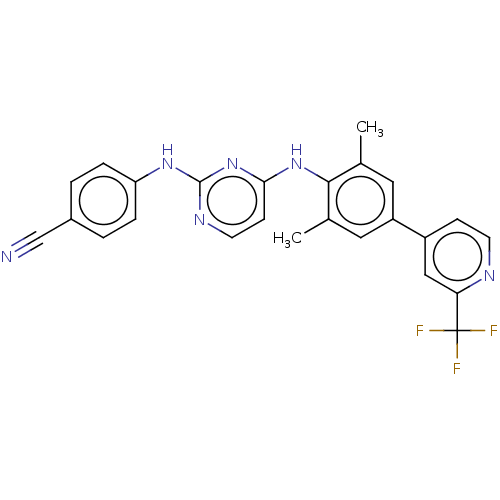 (CHEMBL5091293) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584631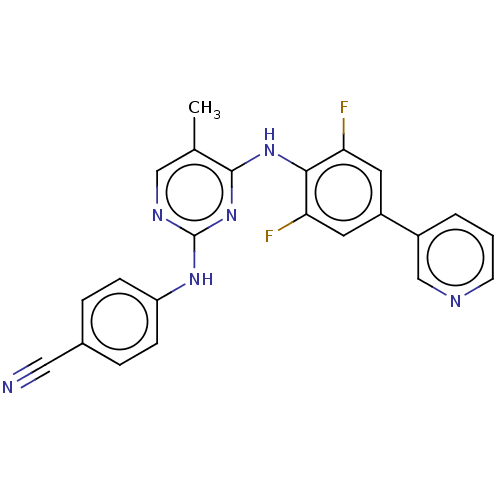 (CHEMBL5092364) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50207551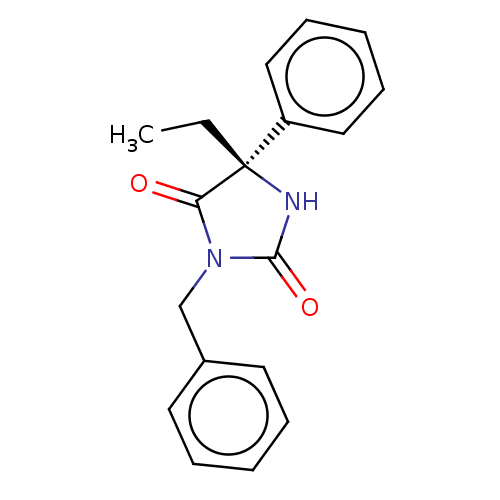 (CHEMBL3977345) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate incubated for 15 to 45 mins in presence of NADPH | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584640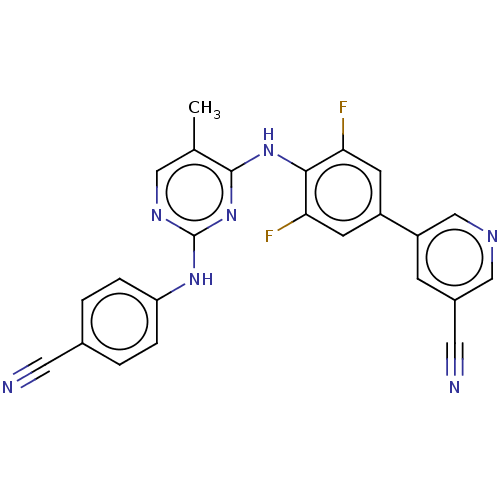 (CHEMBL5078581) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584632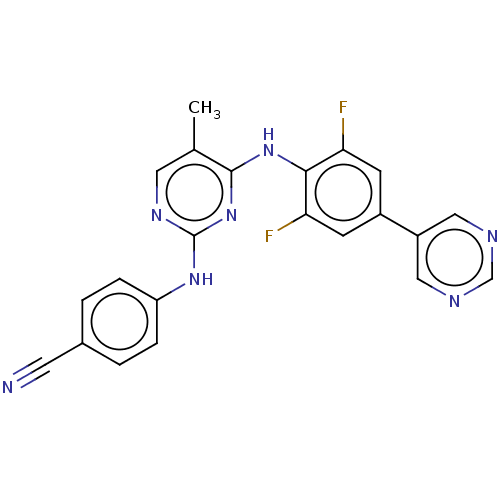 (CHEMBL5088472) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584644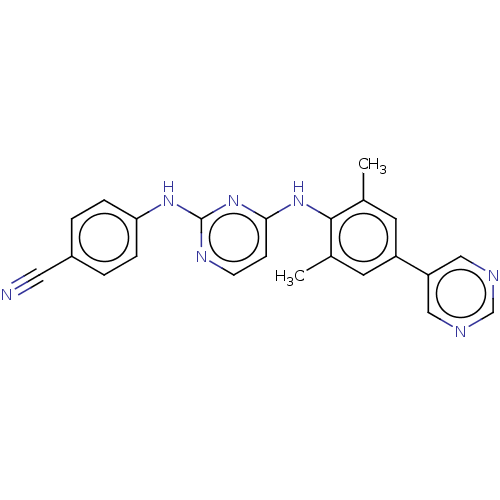 (CHEMBL5091984) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 343 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50584639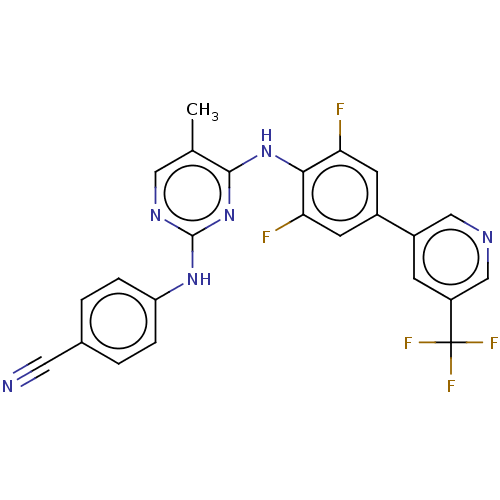 (CHEMBL5079010) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 857 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type p66/p51 HIV1 reverse transcriptase incubated for 40 mins by picogreen dye-based spectrofluorometric analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate incubated for 15 to 45 mins in presence of NADPH | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50584630 (CHEMBL5086160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate incubated for 15 to 45 mins in presence of NADPH | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50584630 (CHEMBL5086160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate incubated for 15 to 45 mins in presence of NADPH | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50584630 (CHEMBL5086160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate incubated for 15 to 45 mins in presence of NADPH | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate incubated for 15 to 45 mins in presence of NADPH | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50584645 (CHEMBL5081783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate incubated for 15 to 45 mins in presence of NADPH | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50584630 (CHEMBL5086160) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate incubated for 15 to 45 mins in presence of NADPH | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50090677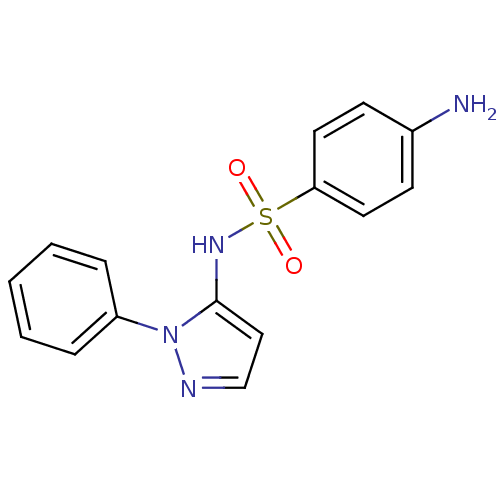 (4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate incubated for 15 to 45 mins in presence of NADPH | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50584645 (CHEMBL5081783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate incubated for 15 to 45 mins in presence of NADPH | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50584645 (CHEMBL5081783) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate incubated for 15 to 45 mins in presence of NADPH | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50584645 (CHEMBL5081783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate incubated for 15 to 45 mins in presence of NADPH | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01676 BindingDB Entry DOI: 10.7270/Q2GH9NT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||