

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378873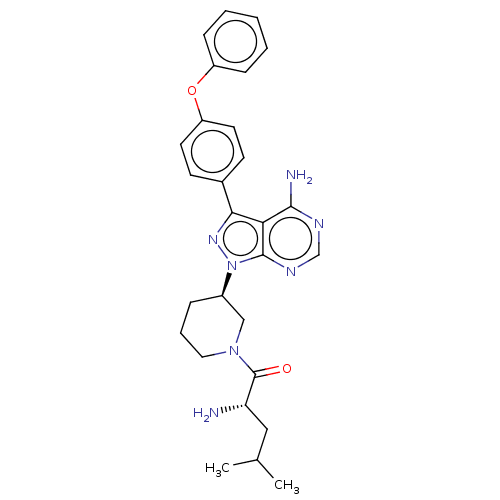 (US10266535, Compound 12) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50300690 (1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 15.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378877 (US10266535, Compound 76) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378878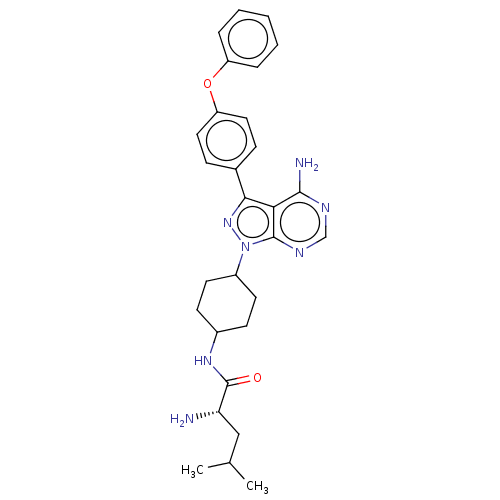 (US10266535, Compound 77) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378857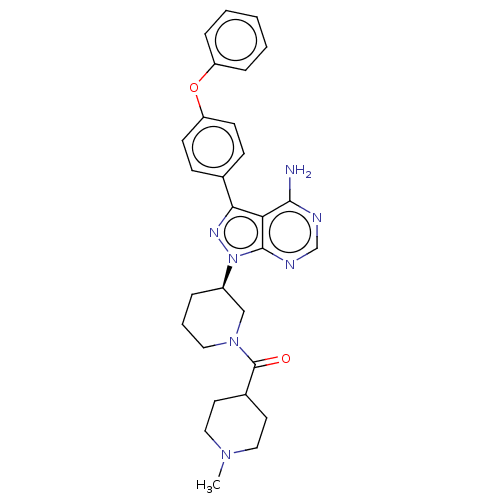 (US10266535, Compound 33) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378878 (US10266535, Compound 77) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378881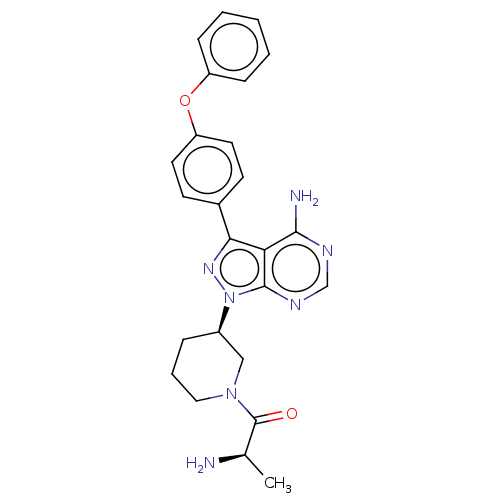 (US10266535, Compound 47) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378868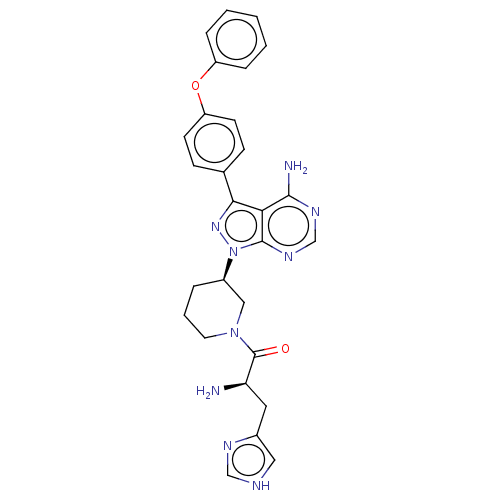 (US10266535, Compound 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378880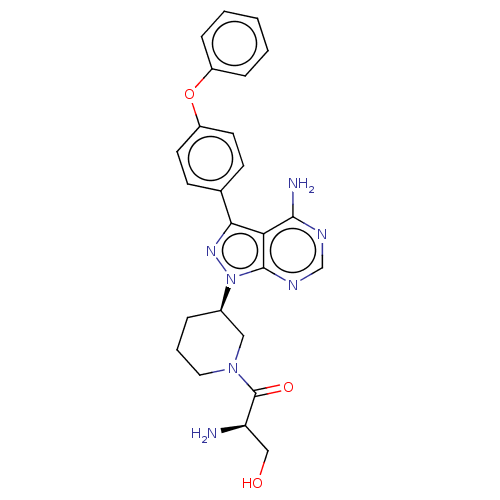 (US10266535, Compound 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378884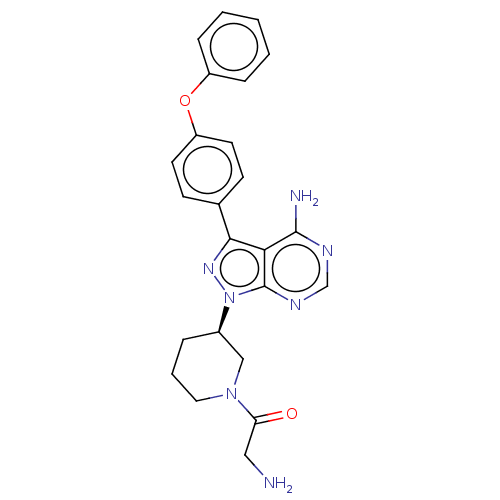 (US10266535, Compound 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378882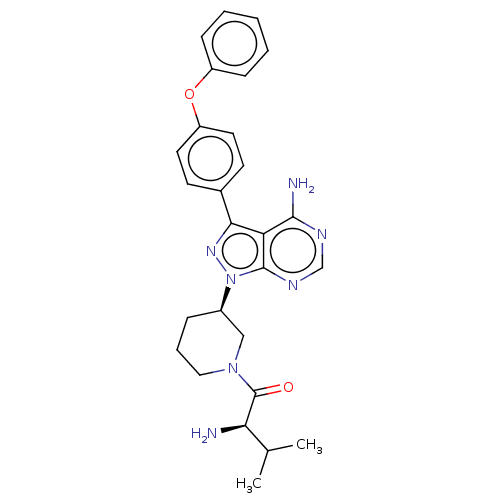 (US10266535, Compound 45) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378884 (US10266535, Compound 43) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378866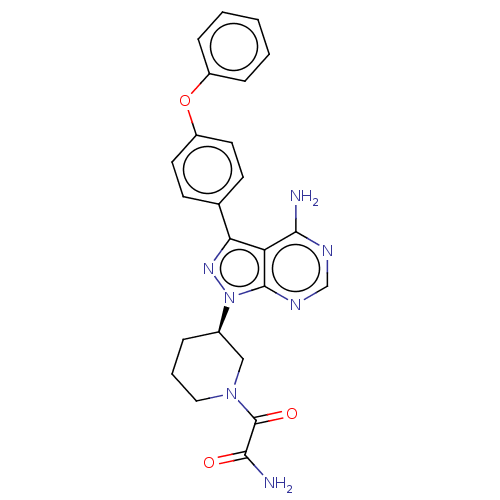 (US10266535, Compound 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378879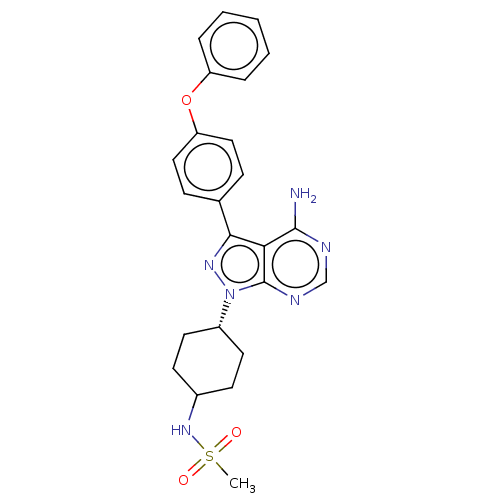 (US10266535, Compound 78) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50135187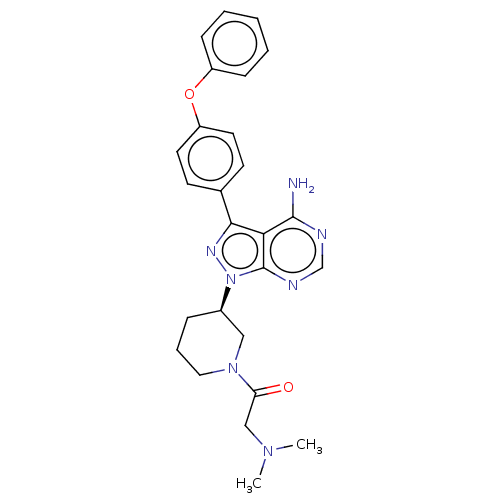 (CHEMBL3745919 | US10266535, Compound 22) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378870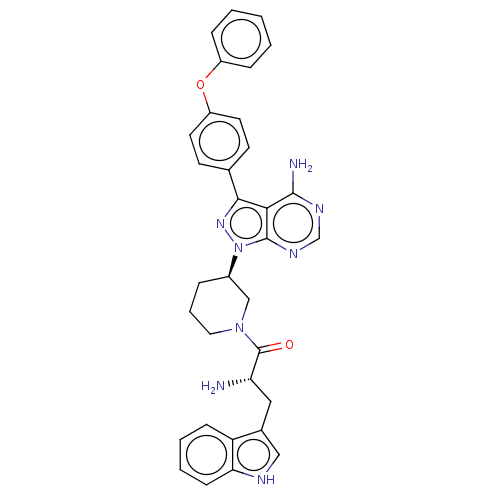 (US10266535, Compound 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378883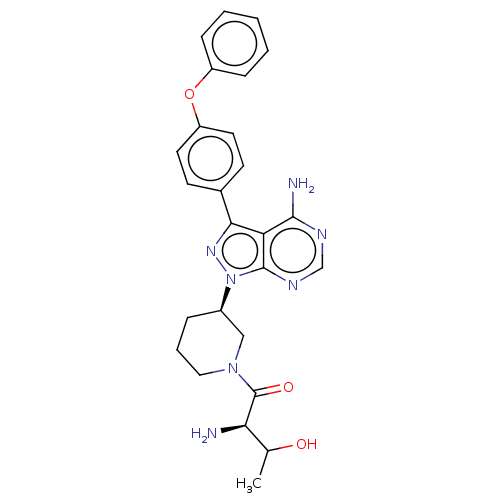 (US10266535, Compound 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378859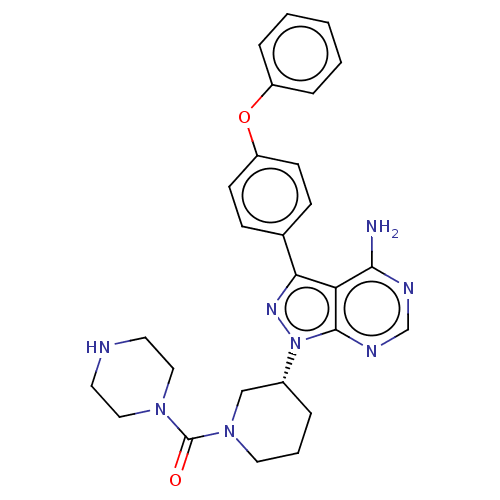 (US10266535, Compound 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378881 (US10266535, Compound 47) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378864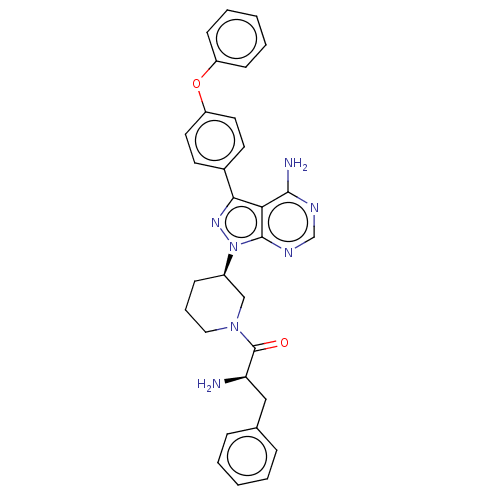 (US10266535, Compound 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378872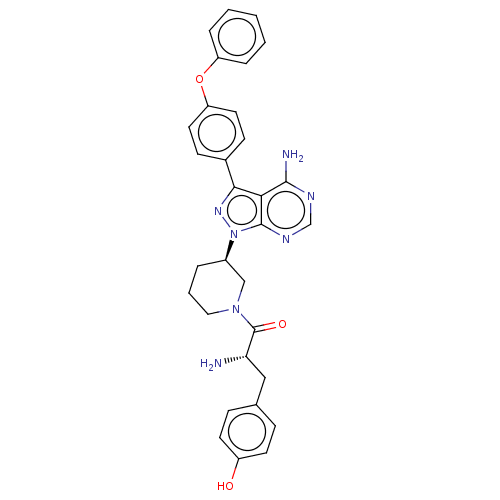 (US10266535, Compound 11) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378860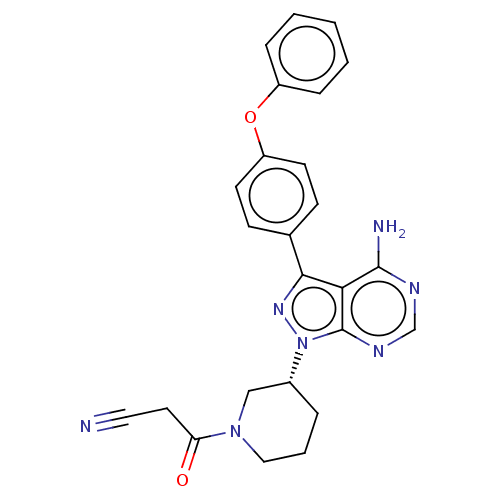 (US10266535, Compound 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378864 (US10266535, Compound 26) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378880 (US10266535, Compound 48) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378858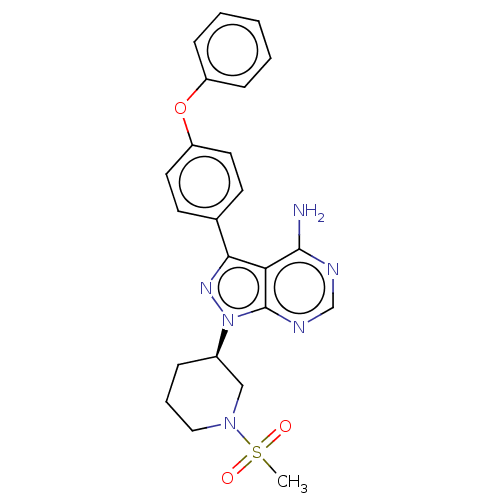 (US10266535, Compound 30) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378878 (US10266535, Compound 77) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378871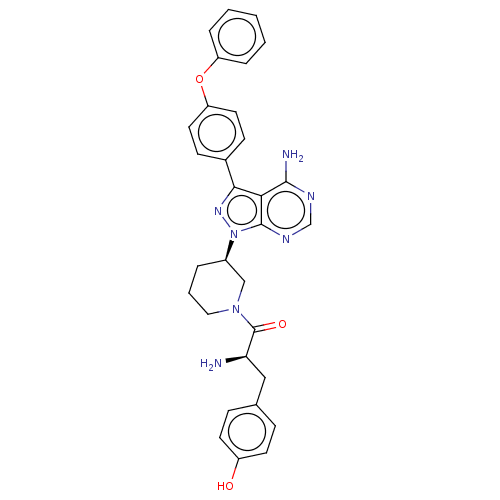 (US10266535, Compound 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378867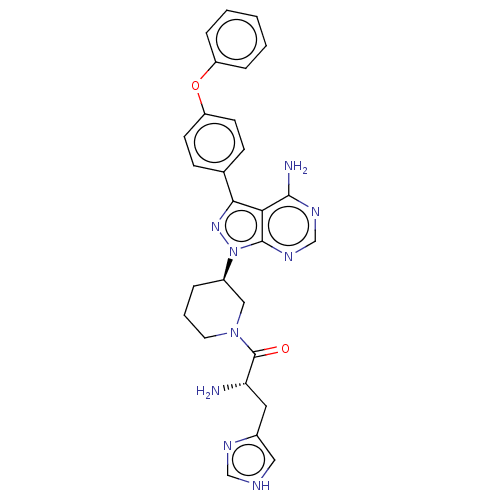 (US10266535, Compound 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378865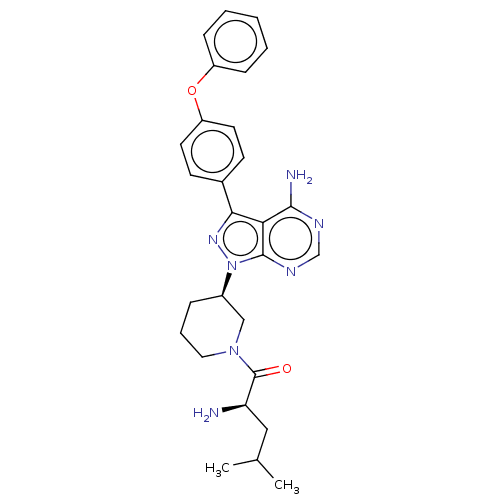 (US10266535, Compound 27) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378871 (US10266535, Compound 10) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378859 (US10266535, Compound 14) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50135187 (CHEMBL3745919 | US10266535, Compound 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378862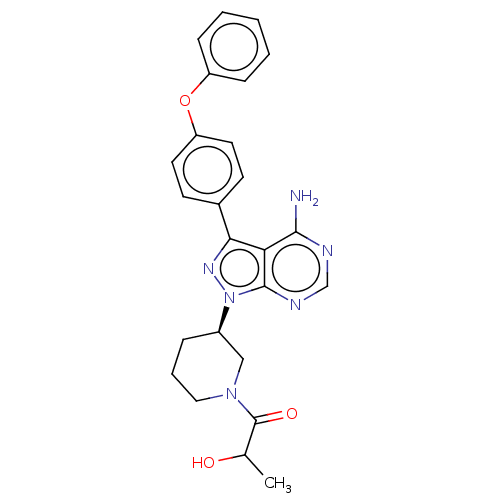 (US10266535, Compound 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378863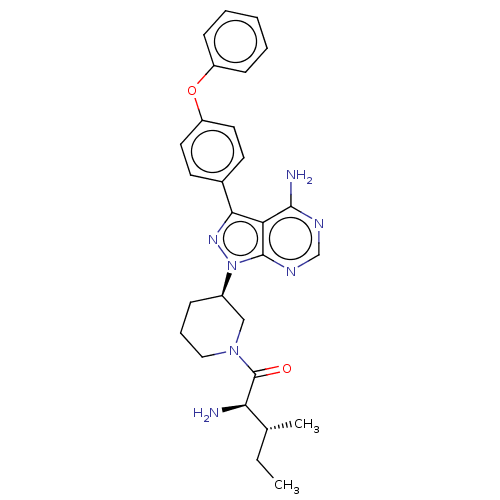 (US10266535, Compound 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378869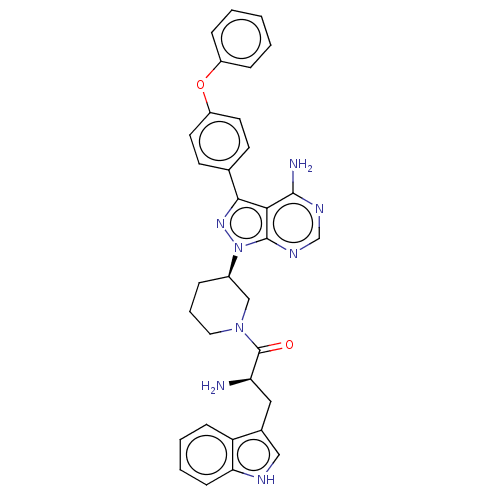 (US10266535, Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378882 (US10266535, Compound 45) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50135187 (CHEMBL3745919 | US10266535, Compound 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378867 (US10266535, Compound 6) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 343 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378879 (US10266535, Compound 78) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 343 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378873 (US10266535, Compound 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 349 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378877 (US10266535, Compound 76) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 388 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378868 (US10266535, Compound 7) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 399 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378866 (US10266535, Compound 5) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 404 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378857 (US10266535, Compound 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 421 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378879 (US10266535, Compound 78) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 455 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378877 (US10266535, Compound 76) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 478 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378874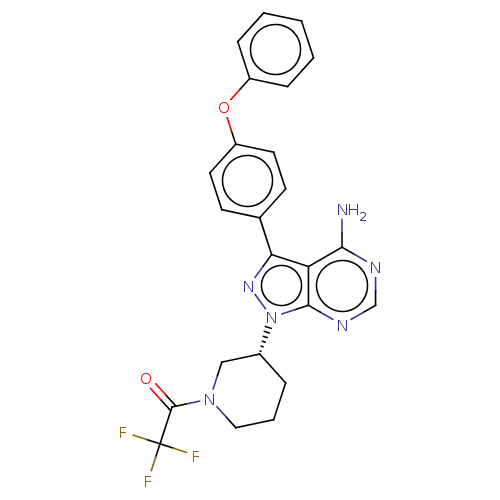 (US10266535, Compound 2) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 572 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378863 (US10266535, Compound 25) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM378865 (US10266535, Compound 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 688 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM378860 (US10266535, Compound 20) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 718 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description In the experiments of in vitro enzymatic activity, the IC50 values of Compound 33, Compound 30, Compound 14, Compound 20, Compound 22, Compound 23, C... | Bioorg Med Chem Lett 16: 2095-100 (2006) BindingDB Entry DOI: 10.7270/Q25141JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |