 Found 174 hits Enz. Inhib. hit(s) with all data for assayid = 1 entry = 6173
Found 174 hits Enz. Inhib. hit(s) with all data for assayid = 1 entry = 6173 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113428
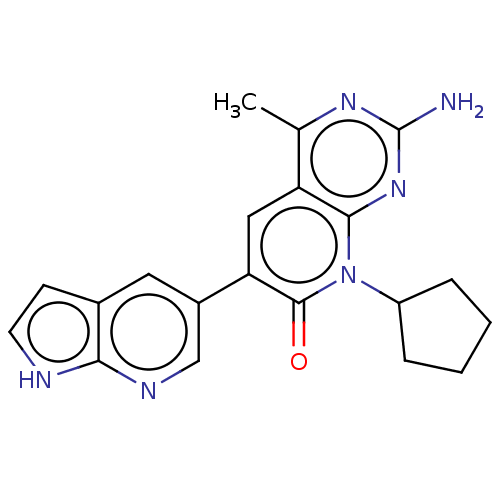
(US8633204, 127)Show SMILES Cc1nc(N)nc2n(C3CCCC3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C20H20N6O/c1-11-15-9-16(13-8-12-6-7-22-17(12)23-10-13)19(27)26(14-4-2-3-5-14)18(15)25-20(21)24-11/h6-10,14H,2-5H2,1H3,(H,22,23)(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433038
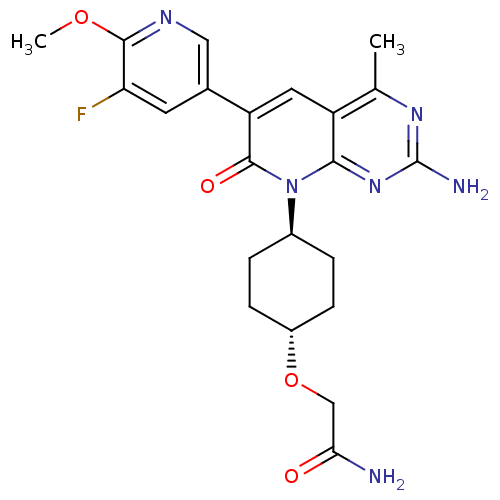
(CHEMBL2375957 | US8633204, 299)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wU:23.28,wD:20.21,(27.28,-44.28,;25.95,-43.51,;24.61,-44.28,;24.61,-45.82,;23.27,-46.58,;21.94,-45.81,;21.94,-44.27,;23.27,-43.5,;23.27,-41.96,;20.6,-46.56,;19.26,-45.78,;17.92,-46.56,;16.58,-45.8,;16.58,-44.26,;15.25,-46.57,;15.25,-48.11,;13.92,-48.88,;16.58,-48.88,;17.91,-48.12,;19.25,-48.89,;19.24,-50.43,;17.9,-51.19,;17.9,-52.72,;19.22,-53.51,;20.56,-52.74,;20.57,-51.2,;19.21,-55.04,;17.88,-55.81,;16.55,-55.03,;15.21,-55.79,;16.56,-53.49,;20.6,-48.12,;21.93,-48.89,)| Show InChI InChI=1S/C22H25FN6O4/c1-11-15-8-16(12-7-17(23)20(32-2)26-9-12)21(31)29(19(15)28-22(25)27-11)13-3-5-14(6-4-13)33-10-18(24)30/h7-9,13-14H,3-6,10H2,1-2H3,(H2,24,30)(H2,25,27,28)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.686 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113443
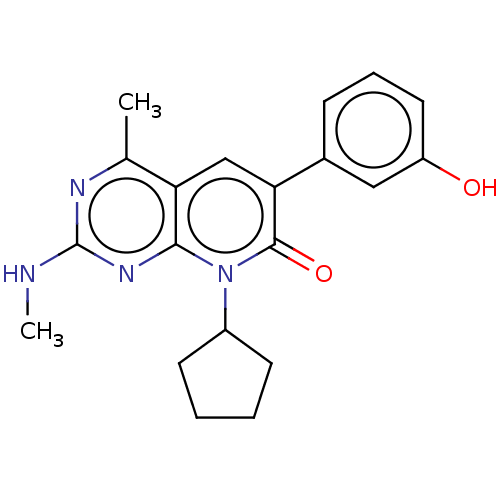
(US8633204, 146)Show SMILES CNc1nc(C)c2cc(-c3cccc(O)c3)c(=O)n(C3CCCC3)c2n1 Show InChI InChI=1S/C20H22N4O2/c1-12-16-11-17(13-6-5-9-15(25)10-13)19(26)24(14-7-3-4-8-14)18(16)23-20(21-2)22-12/h5-6,9-11,14,25H,3-4,7-8H2,1-2H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.731 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113420
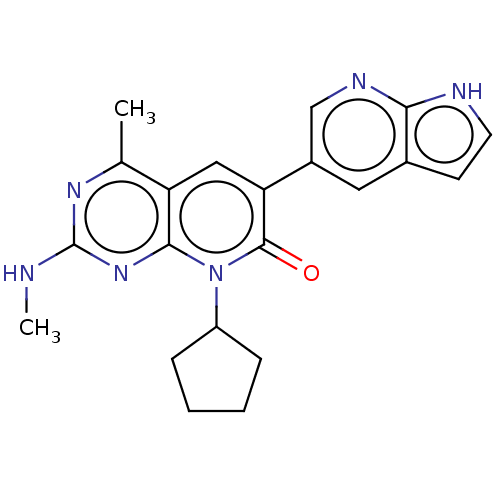
(US8633204, 115)Show SMILES CNc1nc(C)c2cc(-c3cnc4[nH]ccc4c3)c(=O)n(C3CCCC3)c2n1 Show InChI InChI=1S/C21H22N6O/c1-12-16-10-17(14-9-13-7-8-23-18(13)24-11-14)20(28)27(15-5-3-4-6-15)19(16)26-21(22-2)25-12/h7-11,15H,3-6H2,1-2H3,(H,23,24)(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113542

(US8633204, 276)Show SMILES CCNc1nc(C)c2cc(-c3cnc(OC)c(F)c3)c(=O)n([C@H]3CC[C@H](O)CC3)c2n1 |r,wU:22.22,wD:25.26,(-7.96,.31,;-6.47,.7,;-5.38,-.39,;-4.05,.39,;-4.05,1.92,;-2.71,2.69,;-2.71,4.24,;-1.38,1.92,;-.05,2.69,;1.29,1.92,;2.62,2.69,;3.96,1.92,;5.29,2.69,;5.29,4.24,;6.62,5,;7.96,4.24,;3.96,5,;3.96,6.54,;2.62,4.24,;1.29,.39,;2.62,-.39,;-.05,-.39,;-.05,-1.92,;1.29,-2.69,;1.29,-4.24,;-.05,-5,;-.05,-6.54,;-1.38,-4.24,;-1.38,-2.69,;-1.38,.39,;-2.71,-.39,)| Show InChI InChI=1S/C22H26FN5O3/c1-4-24-22-26-12(2)16-10-17(13-9-18(23)20(31-3)25-11-13)21(30)28(19(16)27-22)14-5-7-15(29)8-6-14/h9-11,14-15,29H,4-8H2,1-3H3,(H,24,26,27)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.788 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113434

(US8633204, 137)Show SMILES COc1cc(ccc1O)-c1cc2c(C)nc(N)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C20H22N4O3/c1-11-14-10-15(12-7-8-16(25)17(9-12)27-2)19(26)24(13-5-3-4-6-13)18(14)23-20(21)22-11/h7-10,13,25H,3-6H2,1-2H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.989 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113441
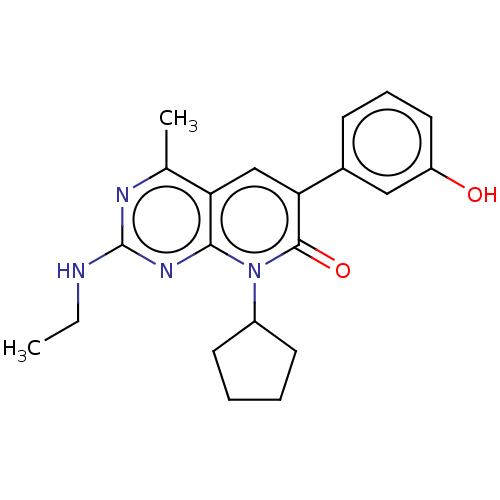
(US8633204, 144)Show SMILES CCNc1nc(C)c2cc(-c3cccc(O)c3)c(=O)n(C3CCCC3)c2n1 Show InChI InChI=1S/C21H24N4O2/c1-3-22-21-23-13(2)17-12-18(14-7-6-10-16(26)11-14)20(27)25(19(17)24-21)15-8-4-5-9-15/h6-7,10-12,15,26H,3-5,8-9H2,1-2H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.01 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113437
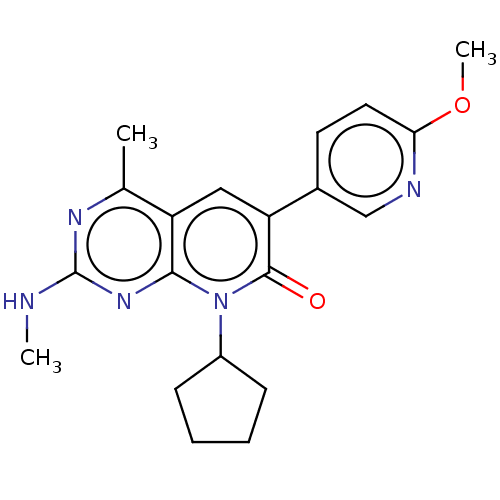
(US8633204, 140)Show SMILES CNc1nc(C)c2cc(-c3ccc(OC)nc3)c(=O)n(C3CCCC3)c2n1 Show InChI InChI=1S/C20H23N5O2/c1-12-15-10-16(13-8-9-17(27-3)22-11-13)19(26)25(14-6-4-5-7-14)18(15)24-20(21-2)23-12/h8-11,14H,4-7H2,1-3H3,(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113479

(US8633204, 191)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:20.21,wD:23.25,(6.67,5,;5.33,5.77,;4,5,;2.67,5.77,;1.33,5,;1.33,3.46,;2.67,2.69,;4,3.46,;5.33,2.69,;,2.69,;-1.33,3.46,;-2.67,2.69,;-4,3.46,;-4,5,;-5.33,2.69,;-5.33,1.15,;-6.67,.39,;-4,.39,;-2.67,1.15,;-1.33,.39,;-1.33,-1.15,;,-1.92,;,-3.46,;-1.33,-4.23,;-1.33,-5.77,;-2.67,-3.46,;-2.67,-1.92,;,1.15,;1.33,.39,)| Show InChI InChI=1S/C20H22FN5O3/c1-10-14-8-15(11-7-16(21)18(29-2)23-9-11)19(28)26(17(14)25-20(22)24-10)12-3-5-13(27)6-4-12/h7-9,12-13,27H,3-6H2,1-2H3,(H2,22,24,25)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113453
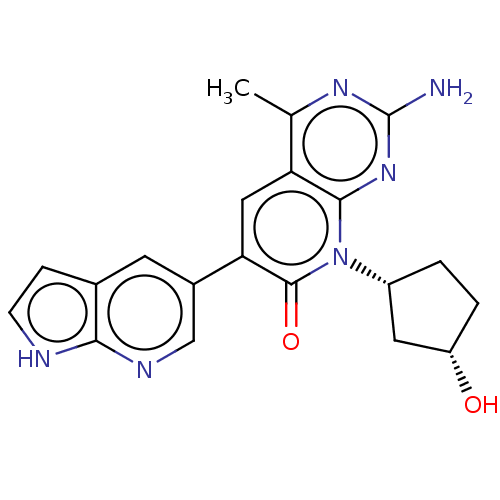
(US8633204, 163)Show SMILES Cc1nc(N)nc2n([C@@H]3CC[C@H](O)C3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 |r| Show InChI InChI=1S/C20H20N6O2/c1-10-15-8-16(12-6-11-4-5-22-17(11)23-9-12)19(28)26(13-2-3-14(27)7-13)18(15)25-20(21)24-10/h4-6,8-9,13-14,27H,2-3,7H2,1H3,(H,22,23)(H2,21,24,25)/t13-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113455
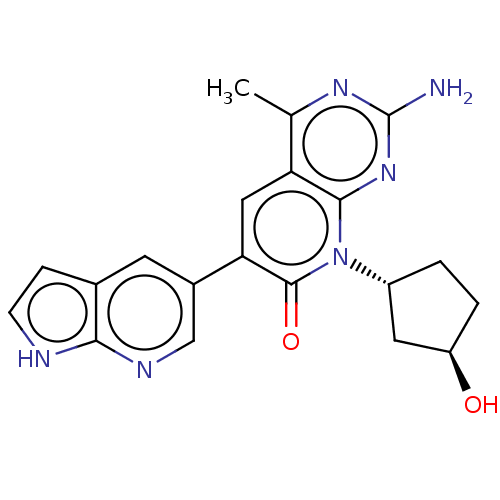
(US8633204, 165)Show SMILES Cc1nc(N)nc2n([C@@H]3CC[C@@H](O)C3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 |r| Show InChI InChI=1S/C20H20N6O2/c1-10-15-8-16(12-6-11-4-5-22-17(11)23-9-12)19(28)26(13-2-3-14(27)7-13)18(15)25-20(21)24-10/h4-6,8-9,13-14,27H,2-3,7H2,1H3,(H,22,23)(H2,21,24,25)/t13-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113544
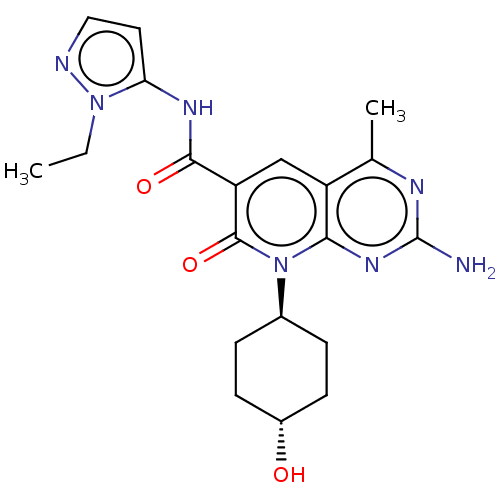
(US8633204, 278)Show SMILES CCn1nccc1NC(=O)c1cc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:21.22,wD:24.26,(2.82,6.54,;2.82,5,;4.15,4.23,;5.61,4.71,;6.52,3.47,;5.61,2.22,;4.15,2.69,;2.82,1.93,;1.48,2.69,;1.48,4.23,;.15,1.93,;-1.18,2.69,;-2.52,1.93,;-3.85,2.69,;-3.85,4.23,;-5.19,1.93,;-5.19,.38,;-6.52,-.38,;-3.85,-.38,;-2.52,.38,;-1.18,-.38,;-1.18,-1.93,;.15,-2.69,;.15,-4.23,;-1.18,-5,;-1.18,-6.54,;-2.52,-4.23,;-2.52,-2.69,;.15,.38,;1.48,-.38,)| Show InChI InChI=1S/C20H25N7O3/c1-3-26-16(8-9-22-26)24-18(29)15-10-14-11(2)23-20(21)25-17(14)27(19(15)30)12-4-6-13(28)7-5-12/h8-10,12-13,28H,3-7H2,1-2H3,(H,24,29)(H2,21,23,25)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113448
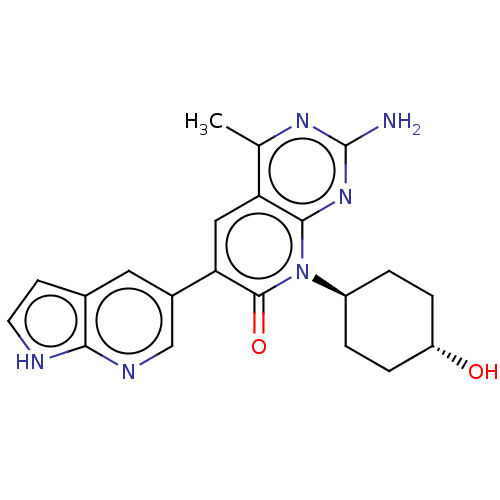
(US8633204, 158)Show SMILES Cc1nc(N)nc2n([C@H]3CC[C@H](O)CC3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 |r,wU:8.7,wD:11.11,(-3.85,4.49,;-3.85,2.95,;-5.19,2.18,;-5.19,.64,;-6.52,-.13,;-3.85,-.13,;-2.52,.64,;-1.18,-.13,;-1.18,-1.67,;.15,-2.44,;.15,-3.98,;-1.18,-4.75,;-1.18,-6.29,;-2.52,-3.98,;-2.52,-2.44,;.15,.64,;1.48,-.13,;.15,2.18,;-1.18,2.95,;-2.52,2.18,;1.48,2.95,;1.48,4.49,;2.82,5.26,;4.15,4.49,;5.61,4.96,;6.52,3.72,;5.61,2.47,;4.15,2.95,;2.82,2.18,)| Show InChI InChI=1S/C21H22N6O2/c1-11-16-9-17(13-8-12-6-7-23-18(12)24-10-13)20(29)27(19(16)26-21(22)25-11)14-2-4-15(28)5-3-14/h6-10,14-15,28H,2-5H2,1H3,(H,23,24)(H2,22,25,26)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113535
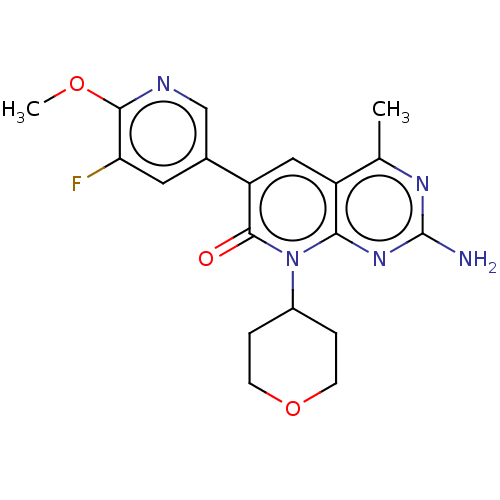
(US8633204, 265)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n(C2CCOCC2)c1=O Show InChI InChI=1S/C19H20FN5O3/c1-10-13-8-14(11-7-15(20)17(27-2)22-9-11)18(26)25(12-3-5-28-6-4-12)16(13)24-19(21)23-10/h7-9,12H,3-6H2,1-2H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.43 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113551
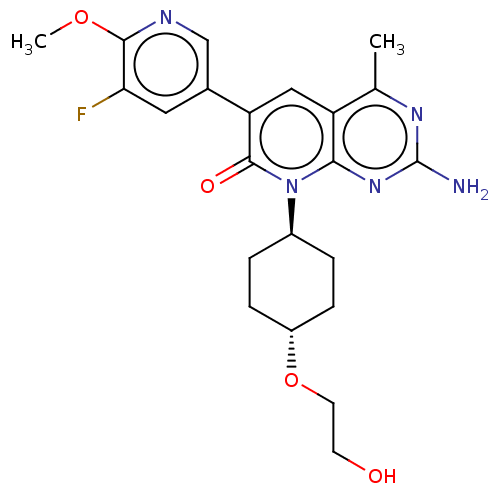
(US8633204, 288)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:20.21,wD:23.28,(6,6.93,;6,5.39,;4.67,4.62,;3.33,5.39,;2,4.62,;2,3.08,;3.33,2.31,;4.67,3.08,;6,2.31,;.67,2.31,;-.67,3.08,;-2,2.31,;-3.33,3.08,;-3.33,4.62,;-4.67,2.31,;-4.67,.77,;-6,,;-3.33,,;-2,.77,;-.67,,;-.67,-1.54,;.67,-2.31,;.67,-3.85,;-.67,-4.62,;-2,-3.85,;-2,-2.31,;-.67,-6.16,;.67,-6.93,;2,-6.16,;3.33,-6.93,;.67,.77,;2,,)| Show InChI InChI=1S/C22H26FN5O4/c1-12-16-10-17(13-9-18(23)20(31-2)25-11-13)21(30)28(19(16)27-22(24)26-12)14-3-5-15(6-4-14)32-8-7-29/h9-11,14-15,29H,3-8H2,1-2H3,(H2,24,26,27)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113417
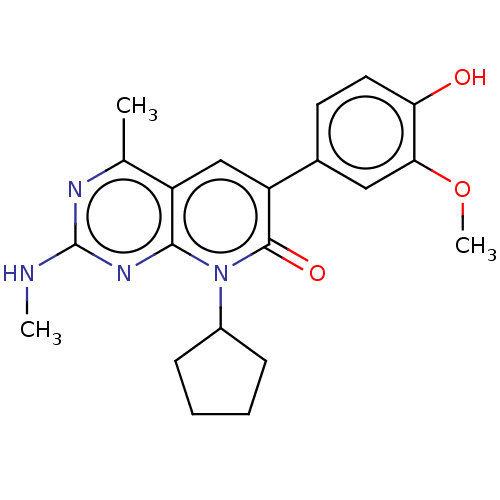
(US8633204, 108)Show SMILES CNc1nc(C)c2cc(-c3ccc(O)c(OC)c3)c(=O)n(C3CCCC3)c2n1 Show InChI InChI=1S/C21H24N4O3/c1-12-15-11-16(13-8-9-17(26)18(10-13)28-3)20(27)25(14-6-4-5-7-14)19(15)24-21(22-2)23-12/h8-11,14,26H,4-7H2,1-3H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.57 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433044
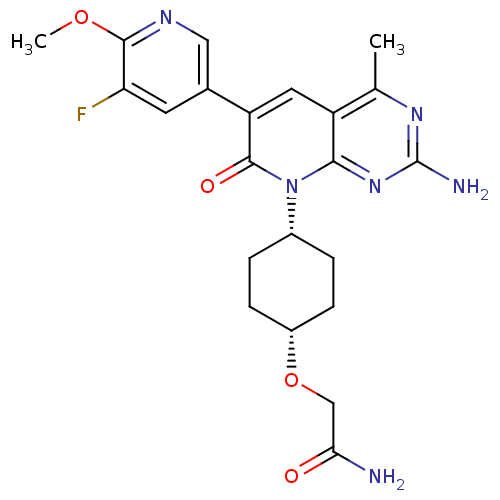
(CHEMBL2375963 | US8633204, 304)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wD:20.21,23.28,(27.43,-30.66,;26.1,-29.89,;24.76,-30.66,;24.76,-32.2,;23.42,-32.96,;22.09,-32.19,;22.09,-30.65,;23.42,-29.88,;23.42,-28.34,;20.75,-32.94,;19.4,-32.16,;18.06,-32.94,;16.73,-32.18,;16.73,-30.64,;15.4,-32.95,;15.4,-34.49,;14.07,-35.26,;16.73,-35.26,;18.06,-34.49,;19.4,-35.27,;19.39,-36.81,;20.72,-37.58,;20.71,-39.12,;19.37,-39.88,;18.05,-39.1,;18.05,-37.57,;19.36,-41.42,;18.02,-42.18,;16.7,-41.41,;15.36,-42.17,;16.71,-39.87,;20.75,-34.5,;22.08,-35.27,)| Show InChI InChI=1S/C22H25FN6O4/c1-11-15-8-16(12-7-17(23)20(32-2)26-9-12)21(31)29(19(15)28-22(25)27-11)13-3-5-14(6-4-13)33-10-18(24)30/h7-9,13-14H,3-6,10H2,1-2H3,(H2,24,30)(H2,25,27,28)/t13-,14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113543

(US8633204, 277)Show SMILES CCNc1nc(C)c2cc(-c3cnc(OC)nc3)c(=O)n([C@H]3CC[C@H](O)CC3)c2n1 |r,wU:21.21,wD:24.25,(-7.96,1.08,;-6.47,1.47,;-5.38,.39,;-4.05,1.15,;-4.05,2.69,;-2.71,3.46,;-2.71,5,;-1.38,2.69,;-.05,3.46,;1.29,2.69,;2.62,3.46,;2.62,5,;3.96,5.77,;5.29,5,;6.62,5.77,;7.96,5,;5.29,3.46,;3.96,2.69,;1.29,1.15,;2.62,.39,;-.05,.39,;-.05,-1.15,;1.29,-1.92,;1.29,-3.46,;-.05,-4.23,;-.05,-5.77,;-1.38,-3.46,;-1.38,-1.92,;-1.38,1.15,;-2.71,.39,)| Show InChI InChI=1S/C21H26N6O3/c1-4-22-20-25-12(2)16-9-17(13-10-23-21(30-3)24-11-13)19(29)27(18(16)26-20)14-5-7-15(28)8-6-14/h9-11,14-15,28H,4-8H2,1-3H3,(H,22,25,26)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.59 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113461
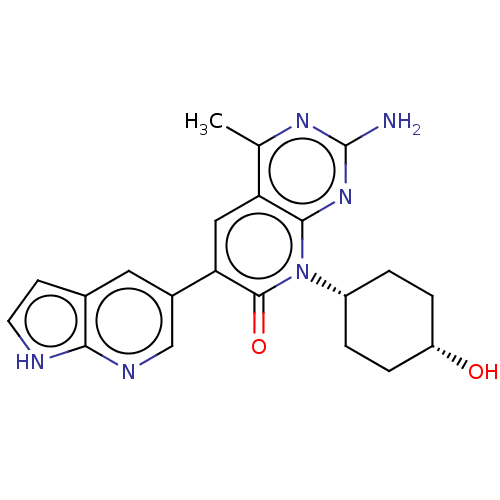
(US8633204, 171)Show SMILES Cc1nc(N)nc2n([C@@H]3CC[C@H](O)CC3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 |r,wU:8.7,11.11,(-3.85,4.49,;-3.85,2.95,;-5.19,2.18,;-5.19,.64,;-6.52,-.13,;-3.85,-.13,;-2.52,.64,;-1.18,-.13,;-1.18,-1.67,;-2.52,-2.44,;-2.52,-3.98,;-1.18,-4.75,;-1.18,-6.29,;.15,-3.98,;.15,-2.44,;.15,.64,;1.48,-.13,;.15,2.18,;-1.18,2.95,;-2.52,2.18,;1.48,2.95,;1.48,4.49,;2.82,5.26,;4.15,4.49,;5.61,4.96,;6.52,3.72,;5.61,2.47,;4.15,2.95,;2.82,2.18,)| Show InChI InChI=1S/C21H22N6O2/c1-11-16-9-17(13-8-12-6-7-23-18(12)24-10-13)20(29)27(19(16)26-21(22)25-11)14-2-4-15(28)5-3-14/h6-10,14-15,28H,2-5H2,1H3,(H,23,24)(H2,22,25,26)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113469

(US8633204, 180)Show SMILES CO[C@H]1CC[C@@H](CC1)n1c2nc(N)nc(C)c2cc(-c2cnc(OC)c(F)c2)c1=O |r,wU:5.8,wD:2.1,(,-6.16,;-1.33,-5.39,;-1.33,-3.85,;,-3.08,;,-1.54,;-1.33,-.77,;-2.67,-1.54,;-2.67,-3.08,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.33,1.54,;-6.67,.77,;-5.33,3.08,;-4,3.85,;-4,5.39,;-2.67,3.08,;-1.33,3.85,;,3.08,;1.33,3.85,;1.33,5.39,;2.67,6.16,;4,5.39,;5.33,6.16,;6.67,5.39,;4,3.85,;5.33,3.08,;2.67,3.08,;,1.54,;1.33,.77,)| Show InChI InChI=1S/C21H24FN5O3/c1-11-15-9-16(12-8-17(22)19(30-3)24-10-12)20(28)27(18(15)26-21(23)25-11)13-4-6-14(29-2)7-5-13/h8-10,13-14H,4-7H2,1-3H3,(H2,23,25,26)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113495
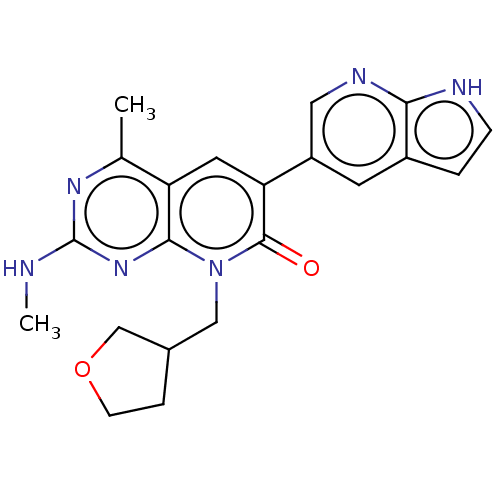
(US8633204, 217)Show SMILES CNc1nc(C)c2cc(-c3cnc4[nH]ccc4c3)c(=O)n(CC3CCOC3)c2n1 Show InChI InChI=1S/C21H22N6O2/c1-12-16-8-17(15-7-14-3-5-23-18(14)24-9-15)20(28)27(10-13-4-6-29-11-13)19(16)26-21(22-2)25-12/h3,5,7-9,13H,4,6,10-11H2,1-2H3,(H,23,24)(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113526
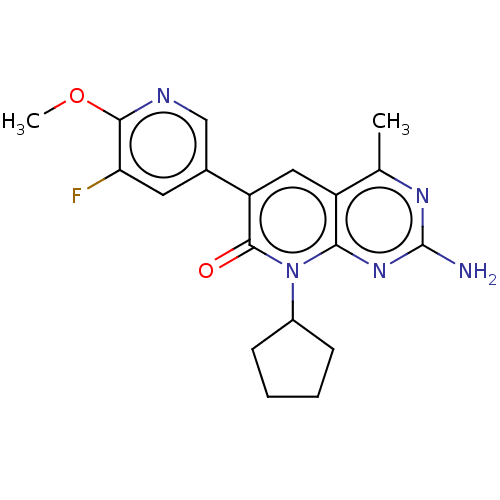
(US8633204, 254)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C19H20FN5O2/c1-10-13-8-14(11-7-15(20)17(27-2)22-9-11)18(26)25(12-5-3-4-6-12)16(13)24-19(21)23-10/h7-9,12H,3-6H2,1-2H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113432

(US8633204, 135)Show SMILES CCNc1nc(C)c2cc(-c3cnc4[nH]ccc4c3)c(=O)n(C3CCCC3)c2n1 Show InChI InChI=1S/C22H24N6O/c1-3-23-22-26-13(2)17-11-18(15-10-14-8-9-24-19(14)25-12-15)21(29)28(20(17)27-22)16-6-4-5-7-16/h8-12,16H,3-7H2,1-2H3,(H,24,25)(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.77 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113541

(US8633204, 274)Show SMILES CNc1nc(C)c2cc(-c3ccc(OC)nc3)c(=O)n([C@H]3CC[C@H](O)CC3)c2n1 |r,wU:20.20,wD:23.24,(-7.34,1.16,;-6,.39,;-4.67,1.16,;-4.67,2.7,;-3.33,3.47,;-3.33,5.01,;-2,2.7,;-.67,3.47,;.67,2.7,;2,3.47,;2,5.01,;3.33,5.78,;4.67,5.01,;6,5.78,;7.34,5.01,;4.67,3.47,;3.33,2.7,;.67,1.16,;2,.39,;-.67,.39,;-.67,-1.16,;.67,-1.93,;.67,-3.47,;-.67,-4.24,;-.67,-5.78,;-2,-3.47,;-2,-1.93,;-2,1.16,;-3.33,.39,)| Show InChI InChI=1S/C21H25N5O3/c1-12-16-10-17(13-4-9-18(29-3)23-11-13)20(28)26(14-5-7-15(27)8-6-14)19(16)25-21(22-2)24-12/h4,9-11,14-15,27H,5-8H2,1-3H3,(H,22,24,25)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113527
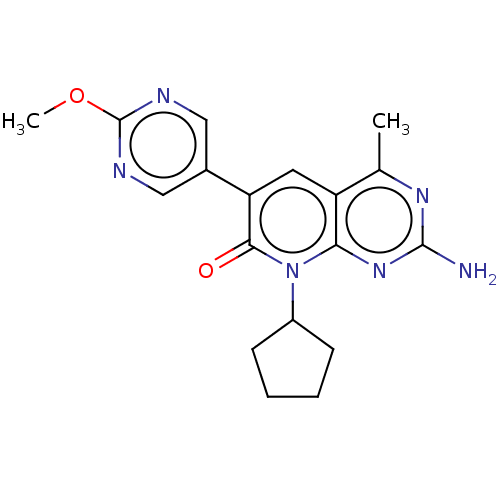
(US8633204, 255)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C18H20N6O2/c1-10-13-7-14(11-8-20-18(26-2)21-9-11)16(25)24(12-5-3-4-6-12)15(13)23-17(19)22-10/h7-9,12H,3-6H2,1-2H3,(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113489
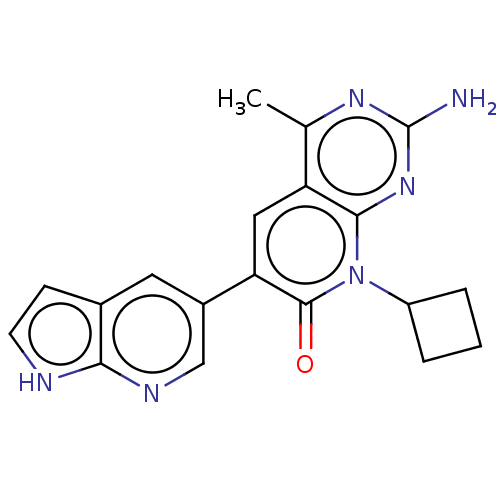
(US8633204, 207)Show SMILES Cc1nc(N)nc2n(C3CCC3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C19H18N6O/c1-10-14-8-15(12-7-11-5-6-21-16(11)22-9-12)18(26)25(13-3-2-4-13)17(14)24-19(20)23-10/h5-9,13H,2-4H2,1H3,(H,21,22)(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113571

(US8633204, 318)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@H]2C[C@@H](C2)OCC(N)=O)c1=O |r,wU:20.21,wD:22.26,(6,6.48,;6,4.94,;4.67,4.17,;3.33,4.94,;2,4.17,;2,2.63,;3.33,1.86,;4.67,2.63,;6,1.86,;.67,1.86,;-.67,2.63,;-2,1.86,;-3.33,2.63,;-3.33,4.17,;-4.67,1.86,;-4.67,.32,;-6,-.45,;-3.33,-.45,;-2,.32,;-.67,-.45,;-.67,-1.99,;.42,-3.08,;-.67,-4.17,;-1.76,-3.08,;-.67,-5.71,;.67,-6.48,;2,-5.71,;3.33,-6.48,;2,-4.17,;.67,.32,;2,-.45,)| Show InChI InChI=1S/C20H21FN6O4/c1-9-13-6-14(10-3-15(21)18(30-2)24-7-10)19(29)27(17(13)26-20(23)25-9)11-4-12(5-11)31-8-16(22)28/h3,6-7,11-12H,4-5,8H2,1-2H3,(H2,22,28)(H2,23,25,26)/t11-,12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.14 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113550
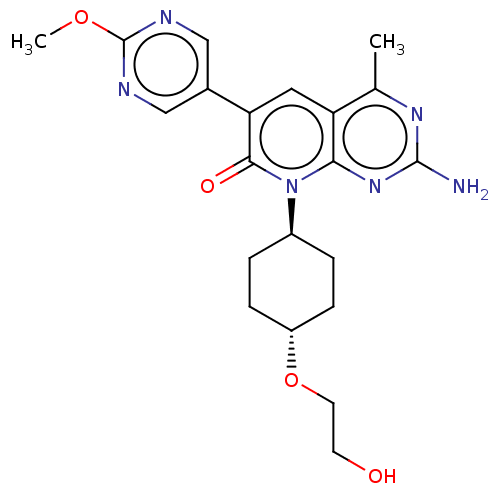
(US8633204, 287)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(6,6.93,;6,5.39,;4.67,4.62,;4.67,3.08,;3.33,2.31,;2,3.08,;2,4.62,;3.33,5.39,;.67,2.31,;-.67,3.08,;-2,2.31,;-3.33,3.08,;-3.33,4.62,;-4.67,2.31,;-4.67,.77,;-6,,;-3.33,,;-2,.77,;-.67,,;-.67,-1.54,;.67,-2.31,;.67,-3.85,;-.67,-4.62,;-2,-3.85,;-2,-2.31,;-.67,-6.16,;.67,-6.93,;2,-6.16,;3.33,-6.93,;.67,.77,;2,,)| Show InChI InChI=1S/C21H26N6O4/c1-12-16-9-17(13-10-23-21(30-2)24-11-13)19(29)27(18(16)26-20(22)25-12)14-3-5-15(6-4-14)31-8-7-28/h9-11,14-15,28H,3-8H2,1-2H3,(H2,22,25,26)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113536

(US8633204, 266)Show InChI InChI=1S/C16H18N6O2/c1-8(2)22-13-11(9(3)20-15(17)21-13)5-12(14(22)23)10-6-18-16(24-4)19-7-10/h5-8H,1-4H3,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113540

(US8633204, 273)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:19.20,wD:22.24,(6.67,5,;5.33,5.77,;4,5,;2.67,5.77,;1.33,5,;1.33,3.46,;2.67,2.69,;4,3.46,;,2.69,;-1.33,3.46,;-2.67,2.69,;-4,3.46,;-4,5,;-5.33,2.69,;-5.33,1.15,;-6.67,.39,;-4,.39,;-2.67,1.15,;-1.33,.39,;-1.33,-1.15,;,-1.92,;,-3.46,;-1.33,-4.23,;-1.33,-5.77,;-2.67,-3.46,;-2.67,-1.92,;,1.15,;1.33,.39,)| Show InChI InChI=1S/C19H22N6O3/c1-10-14-7-15(11-8-21-19(28-2)22-9-11)17(27)25(16(14)24-18(20)23-10)12-3-5-13(26)6-4-12/h7-9,12-13,26H,3-6H2,1-2H3,(H2,20,23,24)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113490

(US8633204, 210)Show SMILES CC(C)n1c2nc(N)nc(C)c2cc(-c2cnc3[nH]ccc3c2)c1=O Show InChI InChI=1S/C18H18N6O/c1-9(2)24-16-13(10(3)22-18(19)23-16)7-14(17(24)25)12-6-11-4-5-20-15(11)21-8-12/h4-9H,1-3H3,(H,20,21)(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113467

(US8633204, 177)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@H](O)CC2)c1=O |r,wU:20.21,23.25,(6,6.54,;6,5,;4.67,4.23,;3.33,5,;2,4.23,;2,2.69,;3.33,1.93,;4.67,2.69,;6,1.93,;.67,1.93,;-.67,2.69,;-2,1.93,;-3.33,2.69,;-3.33,4.23,;-4.67,1.93,;-4.67,.38,;-6,-.38,;-3.33,-.38,;-2,.38,;-.67,-.38,;-.67,-1.93,;-2,-2.69,;-2,-4.23,;-.67,-5,;-.67,-6.54,;.67,-4.23,;.67,-2.69,;.67,.38,;2,-.38,)| Show InChI InChI=1S/C20H22FN5O3/c1-10-14-8-15(11-7-16(21)18(29-2)23-9-11)19(28)26(17(14)25-20(22)24-10)12-3-5-13(27)6-4-12/h7-9,12-13,27H,3-6H2,1-2H3,(H2,22,24,25)/t12-,13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113557

(US8633204, 294)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:20.21,23.28,(6,6.93,;6,5.39,;4.67,4.62,;3.33,5.39,;2,4.62,;2,3.08,;3.33,2.31,;4.67,3.08,;6,2.31,;.67,2.31,;-.67,3.08,;-2,2.31,;-3.33,3.08,;-3.33,4.62,;-4.67,2.31,;-4.67,.77,;-6,,;-3.33,,;-2,.77,;-.67,,;-.67,-1.54,;-2,-2.31,;-2,-3.85,;-.67,-4.62,;.67,-3.85,;.67,-2.31,;-.67,-6.16,;.67,-6.93,;2,-6.16,;3.33,-6.93,;.67,.77,;2,,)| Show InChI InChI=1S/C22H26FN5O4/c1-12-16-10-17(13-9-18(23)20(31-2)25-11-13)21(30)28(19(16)27-22(24)26-12)14-3-5-15(6-4-14)32-8-7-29/h9-11,14-15,29H,3-8H2,1-2H3,(H2,24,26,27)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113477

(US8633204, 189)Show SMILES CCOc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:20.21,wD:23.25,(7.34,5.77,;6,5,;4.67,5.77,;3.33,5,;3.33,3.46,;2,2.69,;.67,3.46,;.67,5,;2,5.77,;-.67,2.69,;-2,3.46,;-3.33,2.69,;-4.67,3.46,;-4.67,5,;-6,2.69,;-6,1.15,;-7.34,.39,;-4.67,.39,;-3.33,1.15,;-2,.39,;-2,-1.15,;-.67,-1.92,;-.67,-3.46,;-2,-4.23,;-2,-5.77,;-3.33,-3.46,;-3.33,-1.92,;-.67,1.15,;.67,.39,)| Show InChI InChI=1S/C21H25N5O3/c1-3-29-18-9-4-13(11-23-18)17-10-16-12(2)24-21(22)25-19(16)26(20(17)28)14-5-7-15(27)8-6-14/h4,9-11,14-15,27H,3,5-8H2,1-2H3,(H2,22,24,25)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113499
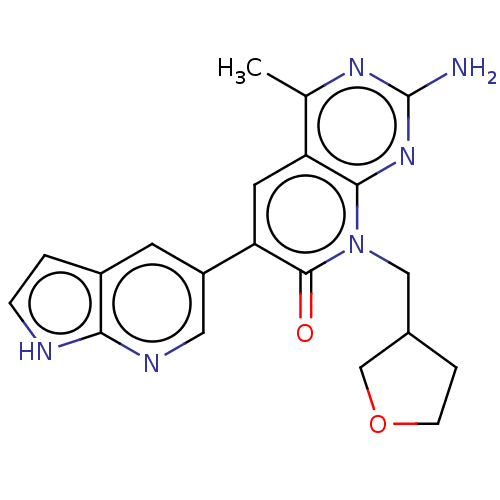
(US8633204, 221)Show SMILES Cc1nc(N)nc2n(CC3CCOC3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C20H20N6O2/c1-11-15-7-16(14-6-13-2-4-22-17(13)23-8-14)19(27)26(9-12-3-5-28-10-12)18(15)25-20(21)24-11/h2,4,6-8,12H,3,5,9-10H2,1H3,(H,22,23)(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113545
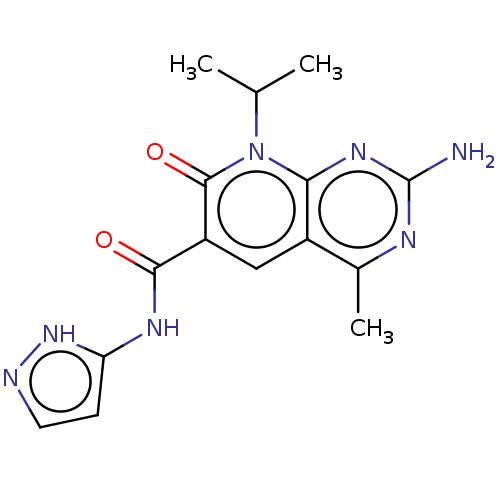
(US8633204, 279)Show SMILES CC(C)n1c2nc(N)nc(C)c2cc(C(=O)Nc2ccn[nH]2)c1=O Show InChI InChI=1S/C15H17N7O2/c1-7(2)22-12-9(8(3)18-15(16)20-12)6-10(14(22)24)13(23)19-11-4-5-17-21-11/h4-7H,1-3H3,(H2,16,18,20)(H2,17,19,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50387590
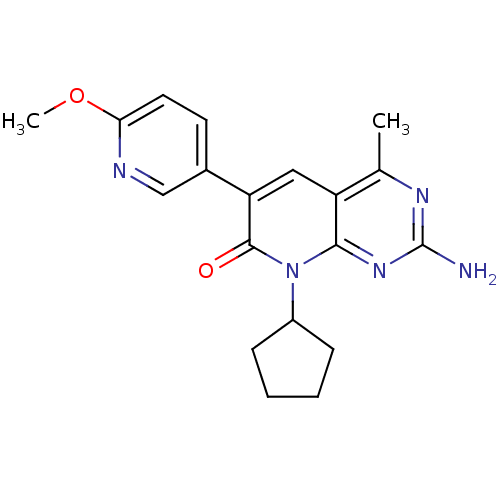
(CHEMBL2057725 | US8633204, 111)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C19H21N5O2/c1-11-14-9-15(12-7-8-16(26-2)21-10-12)18(25)24(13-5-3-4-6-13)17(14)23-19(20)22-11/h7-10,13H,3-6H2,1-2H3,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.96 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113433

(US8633204, 136)Show SMILES CCNc1nc(C)c2cc(-c3ccc(O)c(OC)c3)c(=O)n(C3CCCC3)c2n1 Show InChI InChI=1S/C22H26N4O3/c1-4-23-22-24-13(2)16-12-17(14-9-10-18(27)19(11-14)29-3)21(28)26(20(16)25-22)15-7-5-6-8-15/h9-12,15,27H,4-8H2,1-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.08 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113528
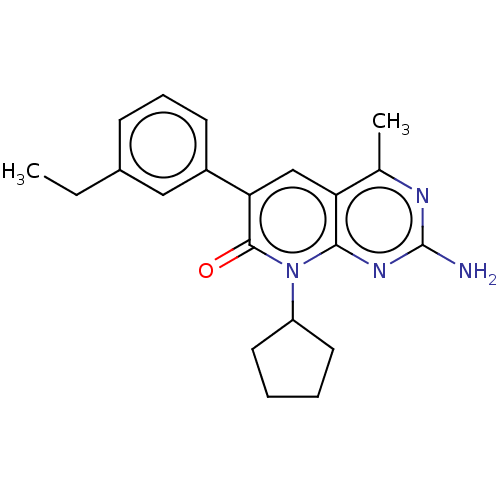
(US8633204, 256)Show SMILES CCc1cccc(c1)-c1cc2c(C)nc(N)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C21H24N4O/c1-3-14-7-6-8-15(11-14)18-12-17-13(2)23-21(22)24-19(17)25(20(18)26)16-9-4-5-10-16/h6-8,11-12,16H,3-5,9-10H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113568
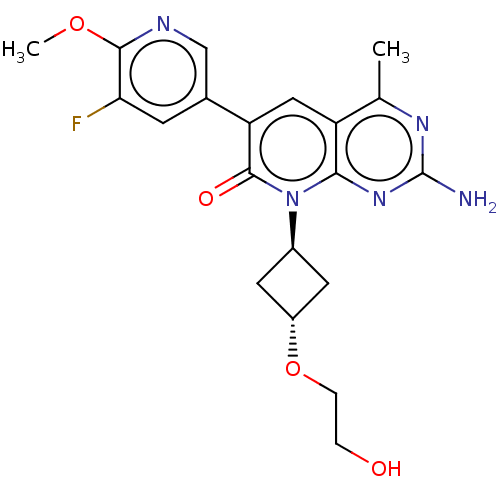
(US8633204, 315)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@H]2C[C@@H](C2)OCCO)c1=O |r,wU:20.21,wD:22.26,(6,6.48,;6,4.94,;4.67,4.17,;3.33,4.94,;2,4.17,;2,2.63,;3.33,1.86,;4.67,2.63,;6,1.86,;.67,1.86,;-.67,2.63,;-2,1.86,;-3.33,2.63,;-3.33,4.17,;-4.67,1.86,;-4.67,.32,;-6,-.45,;-3.33,-.45,;-2,.32,;-.67,-.45,;-.67,-1.99,;.42,-3.08,;-.67,-4.17,;-1.76,-3.08,;-.67,-5.71,;.67,-6.48,;2,-5.71,;3.33,-6.48,;.67,.32,;2,-.45,)| Show InChI InChI=1S/C20H22FN5O4/c1-10-14-8-15(11-5-16(21)18(29-2)23-9-11)19(28)26(17(14)25-20(22)24-10)12-6-13(7-12)30-4-3-27/h5,8-9,12-13,27H,3-4,6-7H2,1-2H3,(H2,22,24,25)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.11 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433043

(CHEMBL2375961 | US8633204, 307)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wD:19.20,22.27,(40.28,-30.68,;38.94,-29.91,;37.61,-30.68,;37.6,-32.22,;36.27,-32.99,;34.94,-32.21,;34.94,-30.68,;36.27,-29.91,;33.6,-32.97,;32.25,-32.19,;30.91,-32.97,;29.58,-32.2,;29.57,-30.66,;28.25,-32.97,;28.25,-34.52,;26.91,-35.28,;29.58,-35.29,;30.91,-34.52,;32.25,-35.3,;32.24,-36.83,;33.57,-37.6,;33.56,-39.15,;32.22,-39.91,;30.89,-39.13,;30.9,-37.6,;32.21,-41.45,;30.87,-42.21,;29.55,-41.43,;28.21,-42.19,;29.56,-39.89,;33.6,-34.52,;34.93,-35.3,)| Show InChI InChI=1S/C21H25N7O4/c1-11-15-7-16(12-8-24-21(31-2)25-9-12)19(30)28(18(15)27-20(23)26-11)13-3-5-14(6-4-13)32-10-17(22)29/h7-9,13-14H,3-6,10H2,1-2H3,(H2,22,29)(H2,23,26,27)/t13-,14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.62 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113485
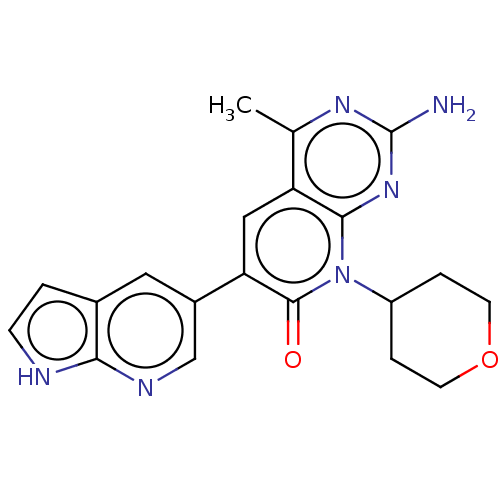
(US8633204, 201)Show SMILES Cc1nc(N)nc2n(C3CCOCC3)c(=O)c(cc12)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C20H20N6O2/c1-11-15-9-16(13-8-12-2-5-22-17(12)23-10-13)19(27)26(14-3-6-28-7-4-14)18(15)25-20(21)24-11/h2,5,8-10,14H,3-4,6-7H2,1H3,(H,22,23)(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433045
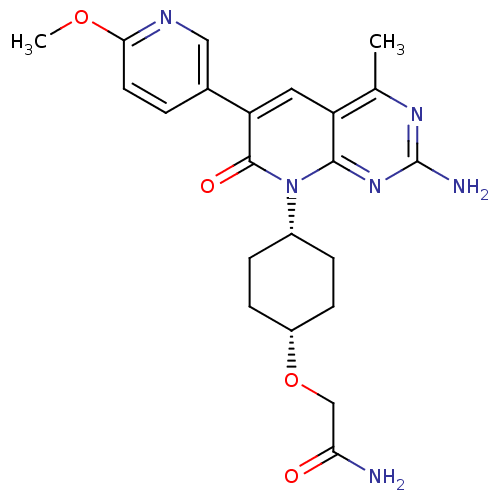
(CHEMBL2375964 | US8633204, 303)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wD:19.20,22.27,(14.55,-30.27,;13.22,-29.49,;11.88,-30.26,;10.54,-29.49,;9.21,-30.26,;9.21,-31.79,;10.54,-32.57,;11.88,-31.8,;7.87,-32.55,;6.53,-31.77,;5.19,-32.55,;3.85,-31.78,;3.85,-30.24,;2.52,-32.55,;2.52,-34.1,;1.19,-34.87,;3.86,-34.87,;5.19,-34.1,;6.52,-34.88,;6.51,-36.41,;7.85,-37.18,;7.83,-38.73,;6.5,-39.49,;5.17,-38.71,;5.18,-37.18,;6.49,-41.03,;5.15,-41.79,;3.82,-41.01,;2.48,-41.77,;3.83,-39.47,;7.87,-34.1,;9.2,-34.88,)| Show InChI InChI=1S/C22H26N6O4/c1-12-16-9-17(13-3-8-19(31-2)25-10-13)21(30)28(20(16)27-22(24)26-12)14-4-6-15(7-5-14)32-11-18(23)29/h3,8-10,14-15H,4-7,11H2,1-2H3,(H2,23,29)(H2,24,26,27)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.87 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113546
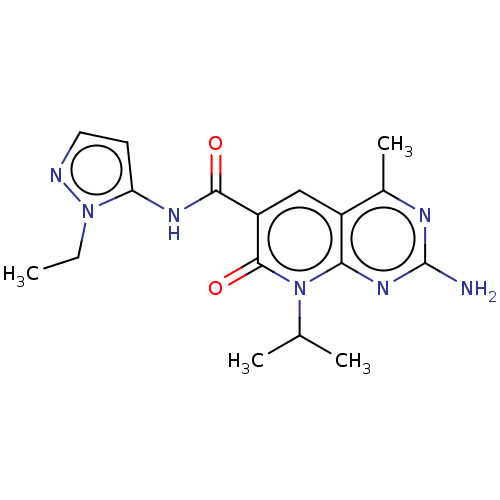
(US8633204, 280)Show SMILES CCn1nccc1NC(=O)c1cc2c(C)nc(N)nc2n(C(C)C)c1=O Show InChI InChI=1S/C17H21N7O2/c1-5-23-13(6-7-19-23)21-15(25)12-8-11-10(4)20-17(18)22-14(11)24(9(2)3)16(12)26/h6-9H,5H2,1-4H3,(H,21,25)(H2,18,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113532
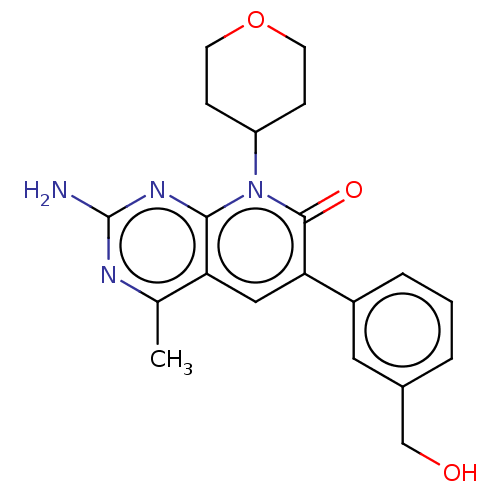
(US8633204, 260)Show SMILES Cc1nc(N)nc2n(C3CCOCC3)c(=O)c(cc12)-c1cccc(CO)c1 Show InChI InChI=1S/C20H22N4O3/c1-12-16-10-17(14-4-2-3-13(9-14)11-25)19(26)24(15-5-7-27-8-6-15)18(16)23-20(21)22-12/h2-4,9-10,15,25H,5-8,11H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113533
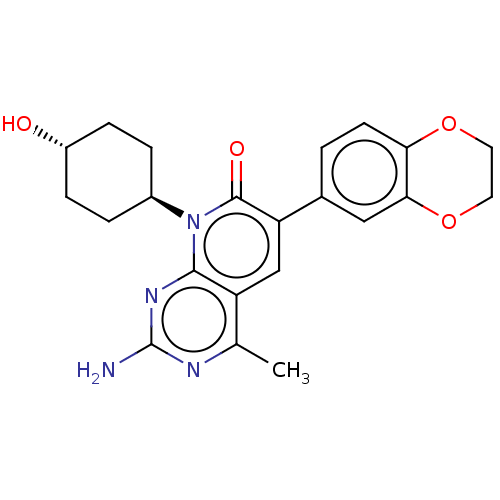
(US8633204, 261)Show SMILES Cc1nc(N)nc2n([C@H]3CC[C@H](O)CC3)c(=O)c(cc12)-c1ccc2OCCOc2c1 |r,wU:8.7,wD:11.11,(-4,5,;-4,3.47,;-5.33,2.69,;-5.33,1.15,;-6.67,.38,;-4,.38,;-2.67,1.15,;-1.33,.38,;-1.33,-1.15,;,-1.93,;,-3.47,;-1.33,-4.23,;-1.33,-5.78,;-2.67,-3.47,;-2.67,-1.93,;,1.15,;1.33,.38,;,2.69,;-1.33,3.47,;-2.67,2.69,;1.33,3.47,;1.33,5,;2.67,5.78,;4,5,;5.33,5.78,;6.67,5,;6.67,3.47,;5.33,2.69,;4,3.47,;2.67,2.69,)| Show InChI InChI=1S/C22H24N4O4/c1-12-16-11-17(13-2-7-18-19(10-13)30-9-8-29-18)21(28)26(20(16)25-22(23)24-12)14-3-5-15(27)6-4-14/h2,7,10-11,14-15,27H,3-6,8-9H2,1H3,(H2,23,24,25)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433037
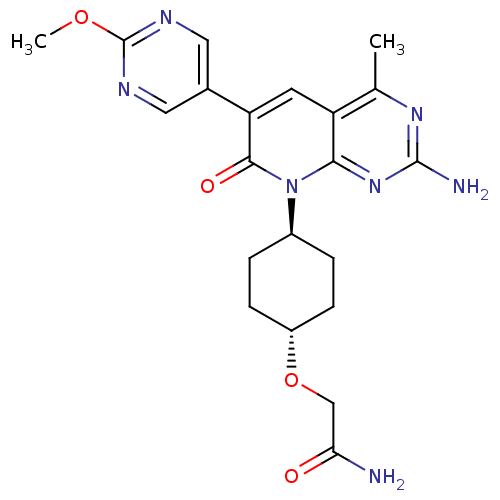
(CHEMBL2375956 | US8633204, 311)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wU:22.27,wD:19.20,(40.72,-44.52,;39.39,-43.75,;38.06,-44.52,;38.05,-46.06,;36.72,-46.82,;35.39,-46.05,;35.39,-44.51,;36.72,-43.74,;34.05,-46.8,;32.7,-46.02,;31.36,-46.8,;30.03,-46.04,;30.02,-44.5,;28.7,-46.81,;28.7,-48.35,;27.36,-49.12,;30.03,-49.12,;31.36,-48.35,;32.7,-49.13,;32.69,-50.67,;31.35,-51.43,;31.34,-52.96,;32.67,-53.74,;34.01,-52.98,;34.02,-51.44,;32.66,-55.28,;31.32,-56.04,;29.99,-55.26,;28.66,-56.03,;30,-53.73,;34.05,-48.36,;35.38,-49.13,)| Show InChI InChI=1S/C21H25N7O4/c1-11-15-7-16(12-8-24-21(31-2)25-9-12)19(30)28(18(15)27-20(23)26-11)13-3-5-14(6-4-13)32-10-17(22)29/h7-9,13-14H,3-6,10H2,1-2H3,(H2,22,29)(H2,23,26,27)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.11 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113465
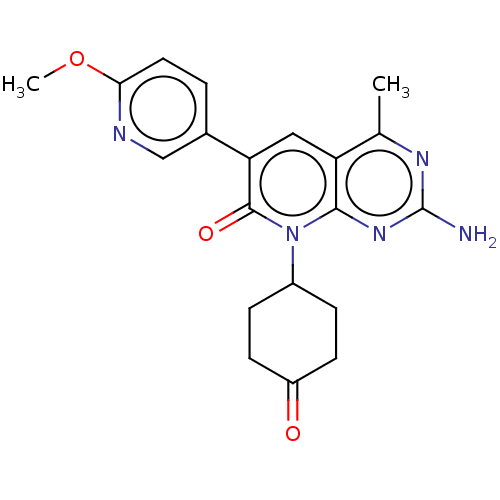
(US8633204, 175)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n(C2CCC(=O)CC2)c1=O Show InChI InChI=1S/C20H21N5O3/c1-11-15-9-16(12-3-8-17(28-2)22-10-12)19(27)25(18(15)24-20(21)23-11)13-4-6-14(26)7-5-13/h3,8-10,13H,4-7H2,1-2H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM113539
(US8633204, 272)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@H](O)CC2)c1=O |r,wU:19.20,22.24,(6.67,5,;5.33,5.77,;4,5,;2.67,5.77,;1.33,5,;1.33,3.46,;2.67,2.69,;4,3.46,;,2.69,;-1.33,3.46,;-2.67,2.69,;-4,3.46,;-4,5,;-5.33,2.69,;-5.33,1.15,;-6.67,.39,;-4,.39,;-2.67,1.15,;-1.33,.39,;-1.33,-1.15,;-2.67,-1.92,;-2.67,-3.46,;-1.33,-4.23,;-1.33,-5.77,;,-3.46,;,-1.92,;,1.15,;1.33,.39,)| Show InChI InChI=1S/C19H22N6O3/c1-10-14-7-15(11-8-21-19(28-2)22-9-11)17(27)25(16(14)24-18(20)23-10)12-3-5-13(26)6-4-12/h7-9,12-13,26H,3-6H2,1-2H3,(H2,20,23,24)/t12-,13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50387582

(CHEMBL2057736 | US8633204, 196)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n(N2CCCC2)c1=O Show InChI InChI=1S/C18H19FN6O2/c1-10-12-8-13(11-7-14(19)16(27-2)21-9-11)17(26)25(24-5-3-4-6-24)15(12)23-18(20)22-10/h7-9H,3-6H2,1-2H3,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kα using an in vitro kinase assay. PI3-Kα activity is measured in... |
US Patent US8633204 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BQT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data