

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50464943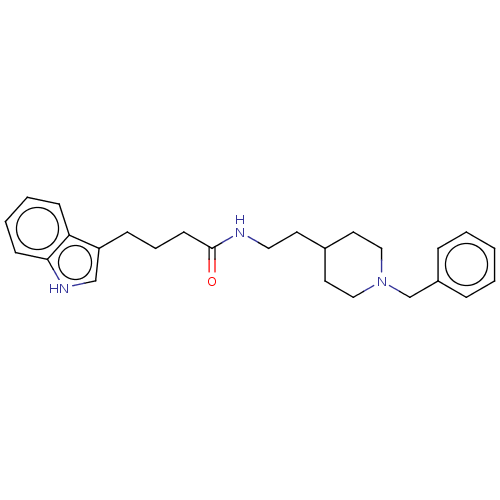 (CHEMBL4294109) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50464944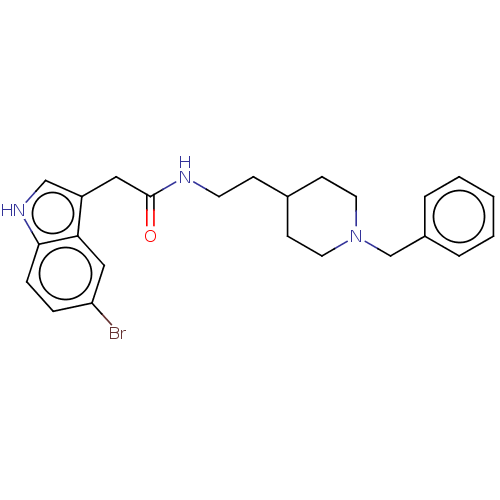 (CHEMBL4285818) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50464942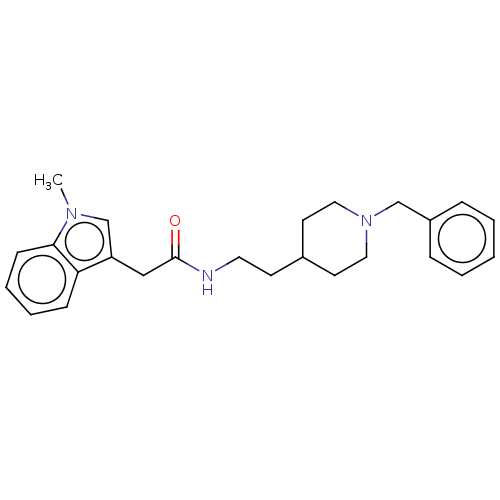 (CHEMBL4293015) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50464946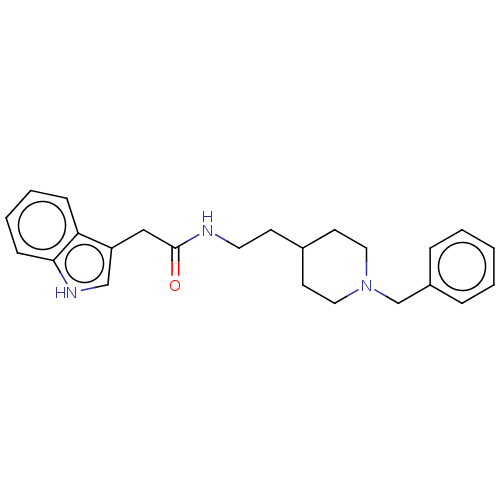 (CHEMBL4283651) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50187201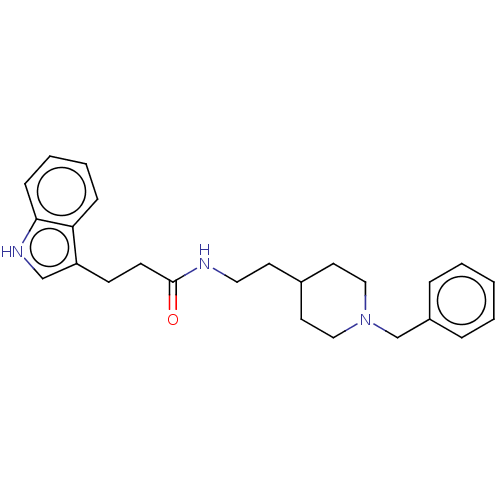 (CHEMBL3824248) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50464945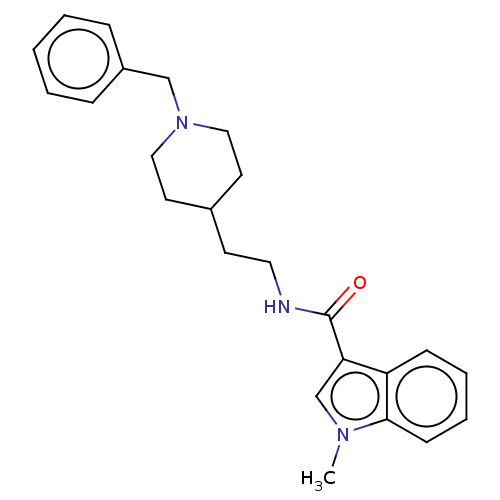 (CHEMBL4282414) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50464947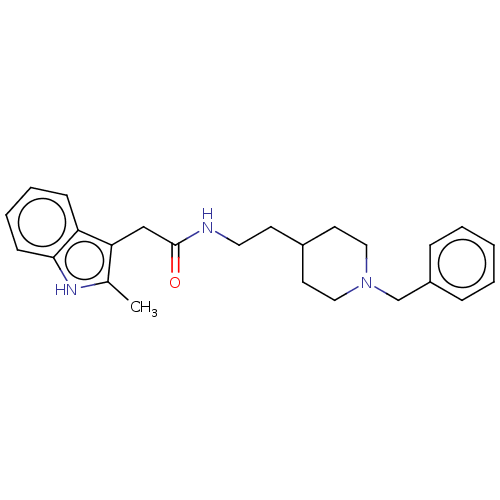 (CHEMBL4277879) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measured for 5 ... | Eur J Med Chem 145: 431-444 (2018) Article DOI: 10.1016/j.ejmech.2018.01.007 BindingDB Entry DOI: 10.7270/Q2JQ13PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||