

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196623 (CHEMBL3976182 | US10167270, Example 00164 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The compounds in DMSO solution (0.5 μL/well) were transferred to a black 96-well plate (the final titration started from 100 μM, a 12 or 24... | US Patent US10501435 (2019) BindingDB Entry DOI: 10.7270/Q24170FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196521 (CHEMBL3939100 | US10167270, Example 00162 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The compounds in DMSO solution (0.5 μL/well) were transferred to a black 96-well plate (the final titration started from 100 μM, a 12 or 24... | US Patent US10501435 (2019) BindingDB Entry DOI: 10.7270/Q24170FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196528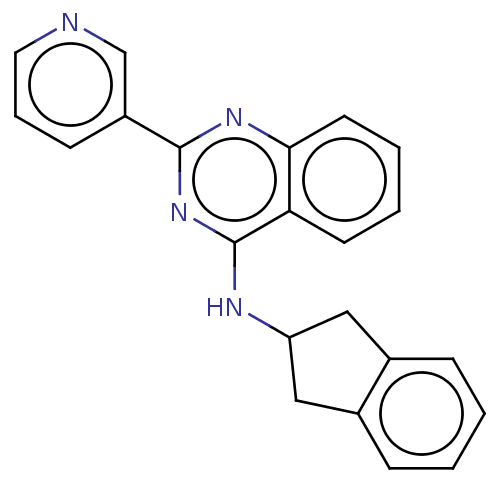 (CHEMBL3923120 | US10167270, Example 00161 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The compounds in DMSO solution (0.5 μL/well) were transferred to a black 96-well plate (the final titration started from 100 μM, a 12 or 24... | US Patent US10501435 (2019) BindingDB Entry DOI: 10.7270/Q24170FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196519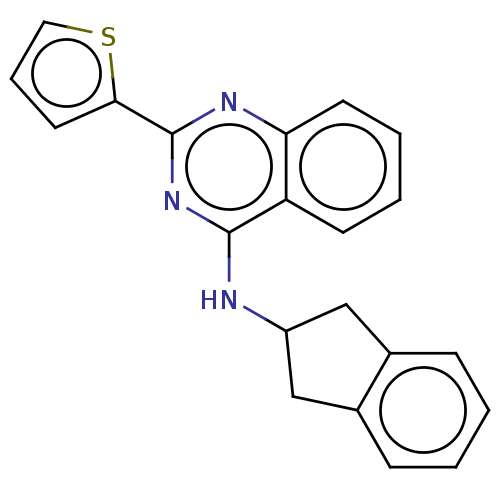 (CHEMBL3930102 | US10167270, Example 00163 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The compounds in DMSO solution (0.5 μL/well) were transferred to a black 96-well plate (the final titration started from 100 μM, a 12 or 24... | US Patent US10501435 (2019) BindingDB Entry DOI: 10.7270/Q24170FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196622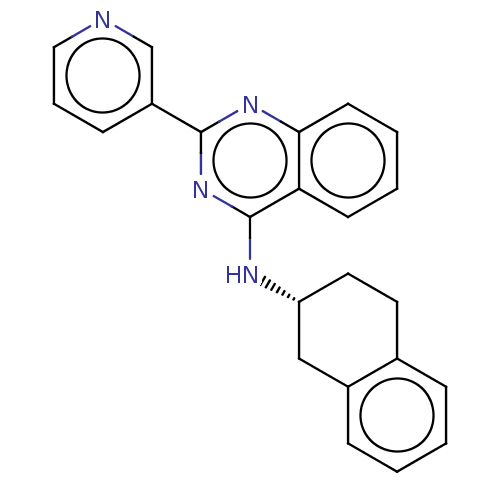 (CHEMBL3923147 | US10167270, Example 00160 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The compounds in DMSO solution (0.5 μL/well) were transferred to a black 96-well plate (the final titration started from 100 μM, a 12 or 24... | US Patent US10501435 (2019) BindingDB Entry DOI: 10.7270/Q24170FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50196622 (CHEMBL3923147 | US10167270, Example 00160 | US1050...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description The compounds in DMSO solution (0.5 μL/well) were transferred to a black 96-well plate (the final titration started from 100 μM, a 12 or 24... | US Patent US10501435 (2019) BindingDB Entry DOI: 10.7270/Q24170FP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||