

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human LOK using RLGRDKYKTLRQIRQ as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human LOK using RLGRDKYKTLRQIRQ as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM93207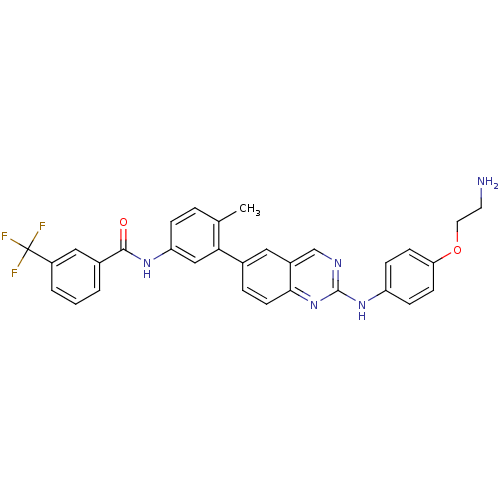 (Kinase inhibitor, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Washington | Assay Description Fluorescence assay used for determination of catch and release efficiency. | ACS Chem Biol 8: 691-9 (2013) Article DOI: 10.1021/cb300623a BindingDB Entry DOI: 10.7270/Q20R9N1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM8226 (3-[(3-chlorophenyl)amino]-4-(2-methoxyphenyl)-2,5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of STK10 (unknown origin) expressed in Sf9 cells assessed as transfer of radiolabelled phosphate group from ATP by reaction biology method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01579 BindingDB Entry DOI: 10.7270/Q2ST7TPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM93207 (Kinase inhibitor, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <20 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Washington | Assay Description Fluorescence assay used for determination of catch and release efficiency. | ACS Chem Biol 8: 691-9 (2013) Article DOI: 10.1021/cb300623a BindingDB Entry DOI: 10.7270/Q20R9N1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM50577328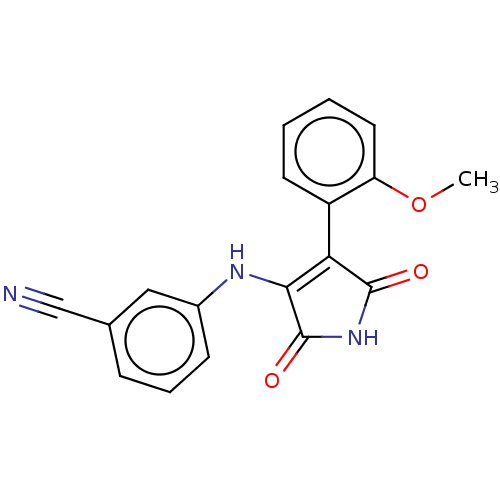 (CHEMBL4875188) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of STK10 (unknown origin) expressed in Sf9 cells assessed as transfer of radiolabelled phosphate group from ATP by reaction biology method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01579 BindingDB Entry DOI: 10.7270/Q2ST7TPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM4552 (4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Molecular Medicine of the Austrian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of STK10 | Leukemia 23: 477-85 (2009) Article DOI: 10.1038/leu.2008.334 BindingDB Entry DOI: 10.7270/Q22Z15R6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM50577368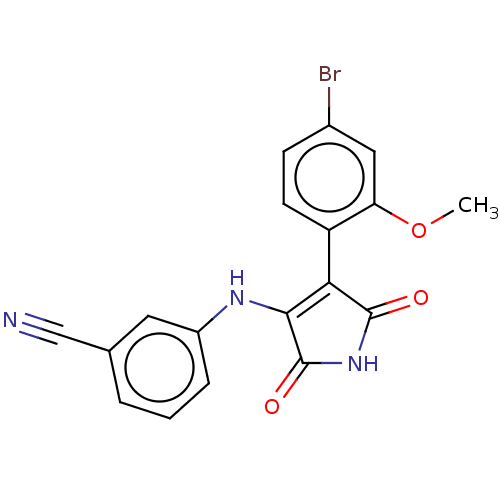 (CHEMBL4878325) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of STK10 (unknown origin) expressed in Sf9 cells assessed as transfer of radiolabelled phosphate group from ATP by reaction biology method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01579 BindingDB Entry DOI: 10.7270/Q2ST7TPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM50577369 (CHEMBL4856751) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of STK10 (unknown origin) expressed in Sf9 cells assessed as transfer of radiolabelled phosphate group from ATP by reaction biology method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01579 BindingDB Entry DOI: 10.7270/Q2ST7TPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM3175 (3-[1-[3-(Amidinothio)propyl]-3-indolyl]-4-(1-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of STK10 (unknown origin) expressed in Sf9 cells assessed as transfer of radiolabelled phosphate group from ATP by reaction biology method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01579 BindingDB Entry DOI: 10.7270/Q2ST7TPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... | J Med Chem 62: 5298-5311 (2019) Article DOI: 10.1021/acs.jmedchem.9b00058 BindingDB Entry DOI: 10.7270/Q24170ZD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM8226 (3-[(3-chlorophenyl)amino]-4-(2-methoxyphenyl)-2,5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of biotinylated full-length STK10 expressed in Escherichia coli DH10Bac assessed as transfer of radiolabelled phosphate from ATP by OMNIA ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01579 BindingDB Entry DOI: 10.7270/Q2ST7TPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM50577340 (CHEMBL4877889) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of STK10 (unknown origin) expressed in Sf9 cells assessed as transfer of radiolabelled phosphate group from ATP by reaction biology method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01579 BindingDB Entry DOI: 10.7270/Q2ST7TPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM50577328 (CHEMBL4875188) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of biotinylated full-length STK10 expressed in Escherichia coli DH10Bac assessed as transfer of radiolabelled phosphate from ATP by OMNIA ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01579 BindingDB Entry DOI: 10.7270/Q2ST7TPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM50577368 (CHEMBL4878325) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of biotinylated full-length STK10 expressed in Escherichia coli DH10Bac assessed as transfer of radiolabelled phosphate from ATP by OMNIA ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01579 BindingDB Entry DOI: 10.7270/Q2ST7TPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM50577369 (CHEMBL4856751) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of biotinylated full-length STK10 expressed in Escherichia coli DH10Bac assessed as transfer of radiolabelled phosphate from ATP by OMNIA ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01579 BindingDB Entry DOI: 10.7270/Q2ST7TPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM50519662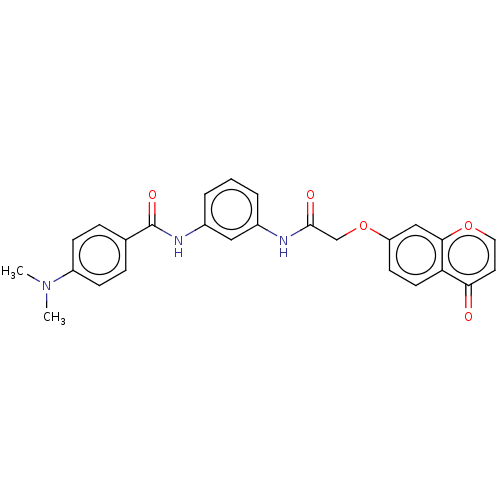 (CHEMBL4438748) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human LOK (1 to 348 residues) using RLGRDKYKTLRQI as substrate after 40 mins in presence of [gamma-33ATP] by radiometric sc... | J Med Chem 62: 10691-10710 (2019) Article DOI: 10.1021/acs.jmedchem.9b01143 BindingDB Entry DOI: 10.7270/Q2MC93FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM50263167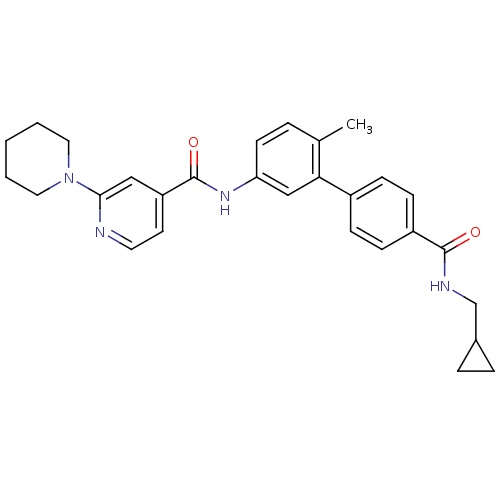 (3,4,5,6-Tetrahydro-2H-[1,2']bipyridinyl-4'-carboxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of LOK (unknown origin) | Bioorg Med Chem Lett 18: 4433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.06.028 BindingDB Entry DOI: 10.7270/Q2JH3M0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM50429867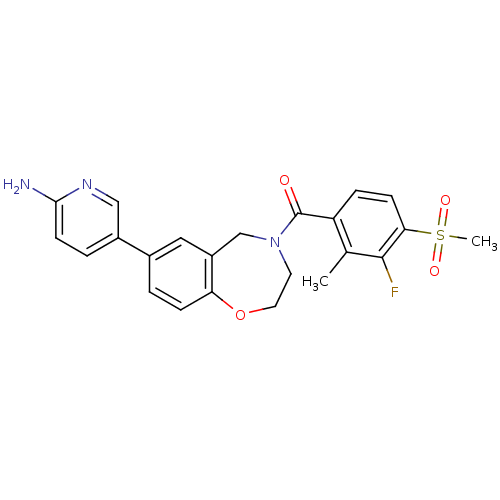 (CHEMBL2333365) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis Curated by ChEMBL | Assay Description Inhibition of LOK (unknown origin) | J Med Chem 56: 2218-34 (2013) Article DOI: 10.1021/jm3007933 BindingDB Entry DOI: 10.7270/Q2ZC847X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM50135286 (CHEMBL3745885) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human LOK using [RLGRDKYKTLRQIRQ] as substrate | Bioorg Med Chem 24: 521-44 (2016) Article DOI: 10.1016/j.bmc.2015.11.045 BindingDB Entry DOI: 10.7270/Q24Q7WT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 10 (Homo sapiens (Human)) | BDBM50577340 (CHEMBL4877889) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of biotinylated full-length STK10 expressed in Escherichia coli DH10Bac assessed as transfer of radiolabelled phosphate from ATP by OMNIA ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01579 BindingDB Entry DOI: 10.7270/Q2ST7TPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||