

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM160935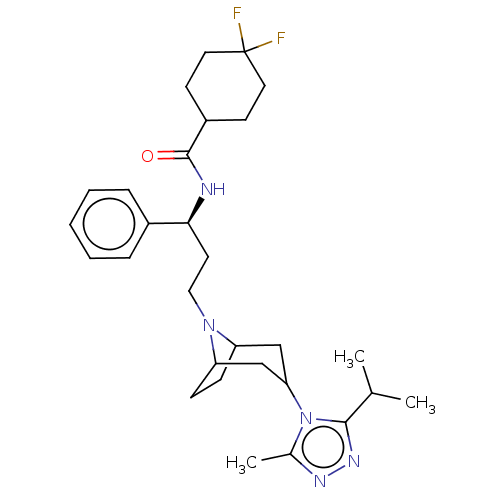 (US10167299, Maraviroc | US9107954, maraviroc) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description Afterwards, the pharmacological profile of bivalent ligand 1 at the chemokine receptor CCR5 was characterized similarly. The competitive radioligand ... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description A bivalent ligand 1 (FIG. 14) that combines the pharmacophores of naltrexone (a MOR antagonist) and maraviroc (a CCR5 antagonist) into one molecule w... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM160933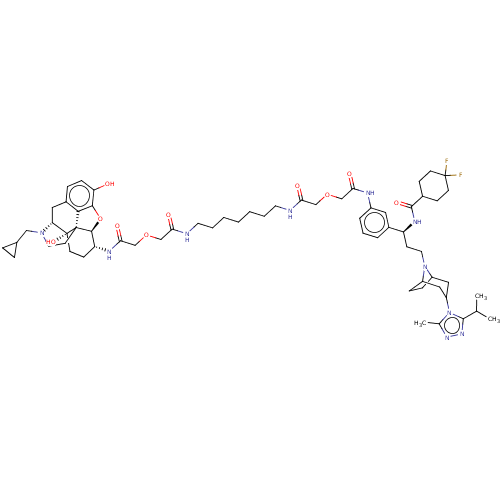 (US9107954, 2) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description A bivalent ligand 1 (FIG. 14) that combines the pharmacophores of naltrexone (a MOR antagonist) and maraviroc (a CCR5 antagonist) into one molecule w... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM160934 (US9107954, 4) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description Afterwards, the pharmacological profile of bivalent ligand 1 at the chemokine receptor CCR5 was characterized similarly. The competitive radioligand ... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM160931 (US9107954, 1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 51.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description A bivalent ligand 1 (FIG. 14) that combines the pharmacophores of naltrexone (a MOR antagonist) and maraviroc (a CCR5 antagonist) into one molecule w... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM160932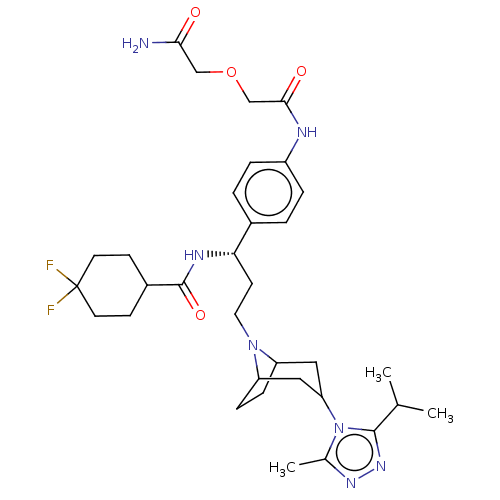 (US9107954, 3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description Afterwards, the pharmacological profile of bivalent ligand 1 at the chemokine receptor CCR5 was characterized similarly. The competitive radioligand ... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM160931 (US9107954, 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description Afterwards, the pharmacological profile of bivalent ligand 1 at the chemokine receptor CCR5 was characterized similarly. The competitive radioligand ... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM160935 (US10167299, Maraviroc | US9107954, maraviroc) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description Then the Ca2+ functional activity of bivalent ligand 1 was evaluated in the Gqi5 transfected CCR5-MOLT-4 cells as described in the literature.3 As ex... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank US Patent | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description As Ca2+ flux is associated with the activation of the MOR, the functional activity of bivalent ligand 1, monovalent ligand 2, and naltrexone was then... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM160934 (US9107954, 4) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description Then the Ca2+ functional activity of bivalent ligand 1 was evaluated in the Gqi5 transfected CCR5-MOLT-4 cells as described in the literature.3 As ex... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM160933 (US9107954, 2) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 37.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description As Ca2+ flux is associated with the activation of the MOR, the functional activity of bivalent ligand 1, monovalent ligand 2, and naltrexone was then... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM160931 (US9107954, 1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description As Ca2+ flux is associated with the activation of the MOR, the functional activity of bivalent ligand 1, monovalent ligand 2, and naltrexone was then... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM160931 (US9107954, 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description Then the Ca2+ functional activity of bivalent ligand 1 was evaluated in the Gqi5 transfected CCR5-MOLT-4 cells as described in the literature.3 As ex... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM160932 (US9107954, 3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 622 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description Then the Ca2+ functional activity of bivalent ligand 1 was evaluated in the Gqi5 transfected CCR5-MOLT-4 cells as described in the literature.3 As ex... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||