

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50416875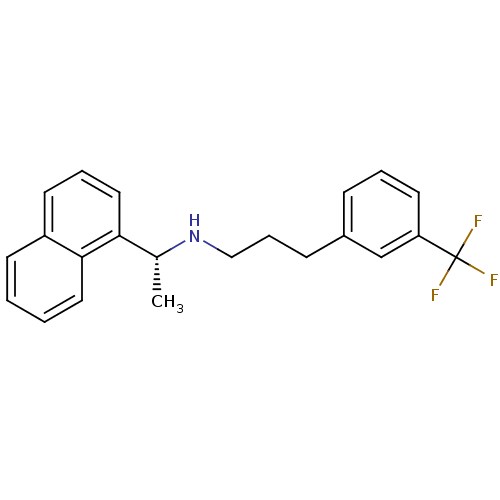 (AMG-073 | AMG073 HCL | CINACALCET | CINACALCET HYD...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S US Patent | Assay Description Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... | US Patent US9487494 (2016) BindingDB Entry DOI: 10.7270/Q21V5CX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM253030 (US9487494, 127 (Compound 1136)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S US Patent | Assay Description Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... | US Patent US9487494 (2016) BindingDB Entry DOI: 10.7270/Q21V5CX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM253032 (US9487494, 53 (Compound 1056)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S US Patent | Assay Description Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... | US Patent US9487494 (2016) BindingDB Entry DOI: 10.7270/Q21V5CX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM253031 (US9487494, 106 (Compound 1115)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S US Patent | Assay Description Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... | US Patent US9487494 (2016) BindingDB Entry DOI: 10.7270/Q21V5CX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM253028 (US9487494, 137 (Compound 1146)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S US Patent | Assay Description Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... | US Patent US9487494 (2016) BindingDB Entry DOI: 10.7270/Q21V5CX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM253029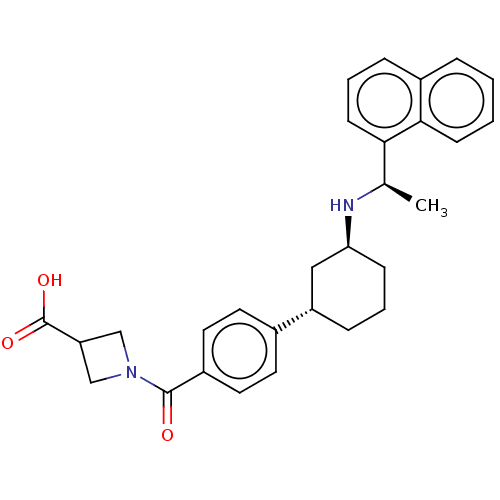 (US9487494, 133 (Compound 1142)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S US Patent | Assay Description Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... | US Patent US9487494 (2016) BindingDB Entry DOI: 10.7270/Q21V5CX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM253025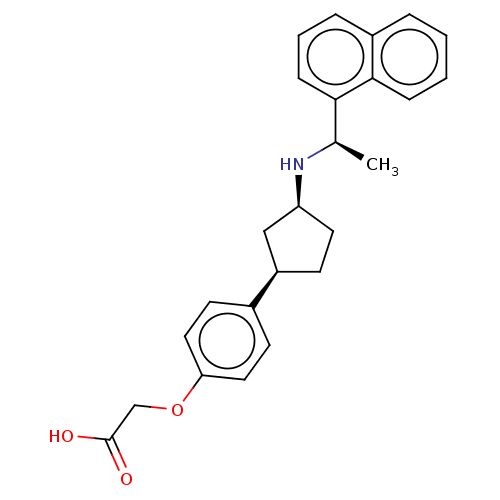 (US9487494, 171 (Compound 1186)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S US Patent | Assay Description Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... | US Patent US9487494 (2016) BindingDB Entry DOI: 10.7270/Q21V5CX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM253026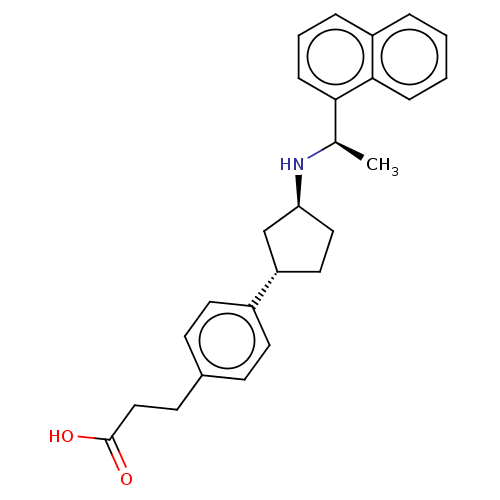 (US9487494, 175 (Compound 1190)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S US Patent | Assay Description Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... | US Patent US9487494 (2016) BindingDB Entry DOI: 10.7270/Q21V5CX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM253027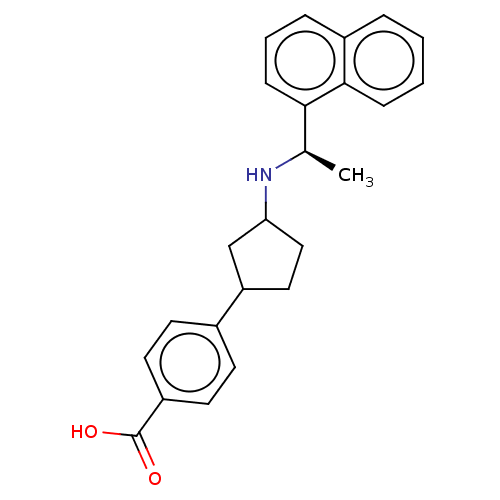 (US9487494, 299 (Compound 1338)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
LEO PHARMA A/S US Patent | Assay Description Incubations were conducted in 96 well microtiter plates based on a method described by BD Biosciences. To the first well in each row, a NADPH regener... | US Patent US9487494 (2016) BindingDB Entry DOI: 10.7270/Q21V5CX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||